Ability of an antibiogram to predict Pseudomonas aeruginosa susceptibility to targeted antimicrobials based on hospital day of isolation - PubMed (original) (raw)
Ability of an antibiogram to predict Pseudomonas aeruginosa susceptibility to targeted antimicrobials based on hospital day of isolation
Deverick J Anderson et al. Infect Control Hosp Epidemiol. 2012 Jun.
Abstract
Objective: To determine the utility of an antibiogram in predicting the susceptibility of Pseudomonas aeruginosa isolates to targeted antimicrobial agents based on the day of hospitalization the specimen was collected.
Design: Single-center retrospective cohort study.
Setting: A 750-bed tertiary care medical center.
Patients and methods: Isolates from consecutive patients with at least 1 clinical culture positive for P. aeruginosa from January 1, 2000, to June 30, 2007, were included. A study antibiogram was created by determining the overall percentages of P. aeruginosa isolates susceptible to amikacin, ceftazidime, ciprofloxacin, gentamicin, imipenem-cilastin, piperacillin-tazobactam, and tobramycin during the study period. Individual logistic regression models were created to determine the day of infection after which the study antibiogram no longer predicted susceptibility to each antibiotic.
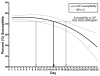
Results: A total of 3,393 isolates were included. The antibiogram became unreliable as a predictor of susceptibility to ceftazidime, imipenem-cilastin, piperacillin-tazobactam, and tobramycin after day 10 and ciprofloxacin after day 15 but longer for gentamicin (day 21) and amikacin (day 28). Time to unreliability of the antibiogram varied for antibiotics based on location of isolation. For example, the time to unreliability of the antibiogram for ceftazidime was 5 days (95% confidence interval [CI], <1-8) in the intensive care unit (ICU) and 12 days (95% CI, 7-21) in non-ICU hospital wards (P = .003).
Conclusions: The ability of the antibiogram to predict susceptibility of P. aeruginosa decreases as duration of hospitalization increases.
Conflict of interest statement
Potential conflicts of interest. D.J.A. has received research funding from Merck and Pfizer, has participated on the speaker’s bureau for Merck, and has received publication royalties from UpToDate Online. R.D. has received research funding from Merck/Schering-Plough, has participated on the speaker’s bureaus for Cubist and Merck/Schering-Plough, and has received publication royalties from UpToDate Online. All other authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
Figures
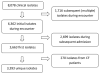
FIGURE 1
Selection of Pseudomonas aeruginosa isolates for inclusion in analyses.
FIGURE 2
Time to unreliability of the antibiogram as a predictor for Pseudomonas aeruginosa susceptibility to ciprofloxacin (CIP).
Similar articles
- A Combination Antibiogram Evaluation for Pseudomonas aeruginosa in Respiratory and Blood Sources from Intensive Care Unit (ICU) and Non-ICU Settings in U.S. Hospitals.
Puzniak L, DePestel DD, Srinivasan A, Ye G, Murray J, Merchant S, DeRyke CA, Gupta V. Puzniak L, et al. Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02564-18. doi: 10.1128/AAC.02564-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30917987 Free PMC article. - Pseudomonas aeruginosa: Evaluation of Pathogen Burden and Drug-Resistance Trends in a Tertiary Care Hospital.
Saeed M, Rasheed F, Afzal RK, Hussain S, Riaz S, Ahmad A. Saeed M, et al. J Coll Physicians Surg Pak. 2018 Apr;28(4):279-283. doi: 10.29271/jcpsp.2018.04.279. J Coll Physicians Surg Pak. 2018. PMID: 29615167 - Development of a combination antibiogram for empirical treatments of Pseudomonas aeruginosa at a university-affiliated teaching hospital.
Ai MY, Lu HE, Lee WY, Liu HY, Chuang HC, Chen BL, Wang EY, Tsao LH, Lee YJ. Ai MY, et al. J Microbiol Immunol Infect. 2023 Apr;56(2):344-350. doi: 10.1016/j.jmii.2022.08.012. Epub 2022 Sep 8. J Microbiol Immunol Infect. 2023. PMID: 36180343 - Imipenem-resistant Pseudomonas aeruginosa: risk factors for nosocomial infections.
Onguru P, Erbay A, Bodur H, Baran G, Akinci E, Balaban N, Cevik MA. Onguru P, et al. J Korean Med Sci. 2008 Dec;23(6):982-7. doi: 10.3346/jkms.2008.23.6.982. Epub 2008 Dec 24. J Korean Med Sci. 2008. PMID: 19119440 Free PMC article. - In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy.
Riou M, Carbonnelle S, Avrain L, Mesaros N, Pirnay JP, Bilocq F, De Vos D, Simon A, Piérard D, Jacobs F, Dediste A, Tulkens PM, Van Bambeke F, Glupczynski Y. Riou M, et al. Int J Antimicrob Agents. 2010 Dec;36(6):513-22. doi: 10.1016/j.ijantimicag.2010.08.005. Int J Antimicrob Agents. 2010. PMID: 20926262
Cited by
- Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis.
Zakhour J, Sharara SL, Hindy JR, Haddad SF, Kanj SS. Zakhour J, et al. Antibiotics (Basel). 2022 Oct 18;11(10):1432. doi: 10.3390/antibiotics11101432. Antibiotics (Basel). 2022. PMID: 36290092 Free PMC article. Review. - Developmental roadmap for antimicrobial susceptibility testing systems.
van Belkum A, Bachmann TT, Lüdke G, Lisby JG, Kahlmeter G, Mohess A, Becker K, Hays JP, Woodford N, Mitsakakis K, Moran-Gilad J, Vila J, Peter H, Rex JH, Dunne WM Jr; JPIAMR AMR-RDT Working Group on Antimicrobial Resistance and Rapid Diagnostic Testing. van Belkum A, et al. Nat Rev Microbiol. 2019 Jan;17(1):51-62. doi: 10.1038/s41579-018-0098-9. Nat Rev Microbiol. 2019. PMID: 30333569 Free PMC article. Review. - A call to action for antimicrobial stewardship in the emergency department: approaches and strategies.
May L, Cosgrove S, L'Archeveque M, Talan DA, Payne P, Jordan J, Rothman RE. May L, et al. Ann Emerg Med. 2013 Jul;62(1):69-77.e2. doi: 10.1016/j.annemergmed.2012.09.002. Epub 2012 Nov 2. Ann Emerg Med. 2013. PMID: 23122955 Free PMC article. Review. - Guidelines on Implementing Antimicrobial Stewardship Programs in Korea.
Yoon YK, Kwon KT, Jeong SJ, Moon C, Kim B, Kiem S, Kim HS, Heo E, Kim SW; Korean Society for Antimicrobial Therapy; Korean Society of Infectious Diseases; Korean Society of Health-System Pharmacist. Yoon YK, et al. Infect Chemother. 2021 Sep;53(3):617-659. doi: 10.3947/ic.2021.0098. Infect Chemother. 2021. PMID: 34623784 Free PMC article. Review. - The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center.
El-Basst R, Saliba S, Saleh L, Saoud N, Azar E, Zalloua P, Chamieh A. El-Basst R, et al. Antibiotics (Basel). 2023 Jan 17;12(2):192. doi: 10.3390/antibiotics12020192. Antibiotics (Basel). 2023. PMID: 36830103 Free PMC article.
References
- Wisplinghoff H, Bischoff T, Tallent S, Seifert H, Wenzel R, Ed-mond M. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. - PubMed
- Pittet D, Li N, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–1078. - PubMed
- Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885–888. - PubMed
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. - PubMed
- Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29:595–607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical