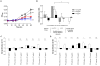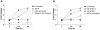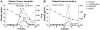Human gastric mucins differently regulate Helicobacter pylori proliferation, gene expression and interactions with host cells - PubMed (original) (raw)
Human gastric mucins differently regulate Helicobacter pylori proliferation, gene expression and interactions with host cells
Emma C Skoog et al. PLoS One. 2012.
Abstract
Helicobacter pylori colonizes the mucus niche of the gastric mucosa and is a risk factor for gastritis, ulcers and cancer. The main components of the mucus layer are heavily glycosylated mucins, to which H. pylori can adhere. Mucin glycosylation differs between individuals and changes during disease. Here we have examined the H. pylori response to purified mucins from a range of tumor and normal human gastric tissue samples. Our results demonstrate that mucins from different individuals differ in how they modulate both proliferation and gene expression of H. pylori. The mucin effect on proliferation varied significantly between samples, and ranged from stimulatory to inhibitory, depending on the type of mucins and the ability of the mucins to bind to H. pylori. Tumor-derived mucins and mucins from the surface mucosa had potential to stimulate proliferation, while gland-derived mucins tended to inhibit proliferation and mucins from healthy uninfected individuals showed little effect. Artificial glycoconjugates containing H. pylori ligands also modulated H. pylori proliferation, albeit to a lesser degree than human mucins. Expression of genes important for the pathogenicity of H. pylori (babA, sabA, cagA, flaA and ureA) appeared co-regulated in response to mucins. The addition of mucins to co-cultures of H. pylori and gastric epithelial cells protected the viability of the cells and modulated the cytokine production in a manner that differed between individuals, was partially dependent of adhesion of H. pylori to the gastric cells, but also revealed that other mucin factors in addition to adhesion are important for H. pylori-induced host signaling. The combined data reveal host-specific effects on proliferation, gene expression and virulence of H. pylori due to the gastric mucin environment, demonstrating a dynamic interplay between the bacterium and its host.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
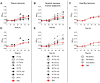
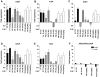
Figure 1. Proliferation of H. pylori J99 in the presence of purified mucins.
Proliferation of H. pylori J99 wt during 60 h in the presence of mucins derived from: A) gastric tumor mucosa at 10 µg/mL (closed symbols = soluble mucins, open symbols = insoluble mucins), B) normal mucosa of tumor-affected stomachs at 10 µg/mL (closed symbols = surface mucins, open symbols = gland mucins), C) healthy mucosa at 10 µg/mL, D) gastric tumor mucosa at 50 µg/mL, E) normal mucosa of tumor-affected stomachs at 50 µg/mL and E) healthy mucosa at 50 µg/mL. Control without mucins (PBS control) is shown in bold red. An OD560 of 0.1 corresponds to 107 CFU/mL.
Figure 2. Concentration- and tissue-dependency of purified mucins for H. pylori J99 proliferation.
A) Concentration-dependency of one stimulatory (P5 TA-AS, black closed symbols) and one inhibitory mucin sample (P7 TA-AG, blue open symbols) on H. pylori proliferation comparing mucin concentrations of 125 (▪), 50 (▴) and 10 (▾) µg/mL to proliferation without mucins (bold red ▪). (***p≤0.001, One-way ANOVA, Dunnett t-test, compared to J99 without mucins after 60 h proliferation.) B) H. pylori fold proliferation level compared to PBS control after 60 h with mucins grouped by tissue type origin at 125, 50 and 10 µg/mL of mucin. (*p = 0.046, One-way ANOVA, Tukey's post hoc test.) C and D) H. pylori fold proliferation level compared to PBS control after 37 h and 60 h with mucins from superficial (white bars) or gland (black bars) mucosal tissue from the same individual. (***p≤0.001, *p<0.05, ns = not significant, Student's t-test.) Although the differences between gland and surface derived mucins was larger at 60 h than at 37 h, some of the growth curves had lost their parabolic growth by this time, which explains why the results for one sample is different at 60 h than at 37 h.
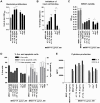
Figure 3. Binding of H. pylori J99 to mucin samples.
Binding of biotinylated H. pylori J99 to mucin samples was measured in a microtiter-based assay. The presence or absence of Leb and sialyl-Lex in each mucin samples, as described in Table 2, is indicated below the x-axis.
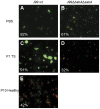
Figure 4. Aggregation and viability of H. pylori J99 after culture with mucins.
H. pylori were stained with LIVE/DEAD® BacLight™ bacterial viability staining after 37 h culture with or without 50 µg/mL of mucin samples. Green represents bacteria with intact membranes and red represents dead bacteria or bacteria with damaged membranes. The percentages of live bacteria are noted in each picture. A) J99 wt with PBS, B) J99Δ_babA_Δ_sabA_ with PBS, C) J99 wt with patient 1 soluble tumor mucins, D) J99Δ_babA_Δ_sabA_ with patient 1 soluble tumor mucins and E) J99 wt with patient 10 healthy gastric mucins.
Figure 5. Proliferation of H. pylori J99 in the presence of HSA-glycoconjugates and recombinant mucins.
A) Proliferation of H. pylori J99 wt cultured with 50 µg/mL of Leb or sialyl-Lex glycoconjugate and HSA control. B) Proliferation of H. pylori J99 wt cultured with 50 µg/mL of recombinant PSGL-1. CM = unmodified CHO-K1 PSGL-1 containing mono- and disialylated core 1, CM-SLex = sialyl-Lex substituted PSGL-1, CM-Leb = Leb substituted PSGL-1, CM-A = blood group A active PSGL-1 and PPM = mannose-containing PSGL-1 lacking sialic acid produced in the yeast Pichia pastoris. C) Fold proliferation level of H. pylori J99 wt in the presence of HSA-glycoconjugates compared to HSA control after 37 h and recombinant PSGL-1 compared to PBS control after 115 h. ***p≤0.001, *p<0.05, Student's t-test. D-F) Bacteria were stained with LIVE/DEAD® BacLight™ bacterial viability kit after culture with D) HSA-Leb conjugate, E) HSA-sialyl-Lex conjugate and F) HSA control. Green represents bacteria with intact membranes and red represents dead bacteria or bacteria with damaged membranes.
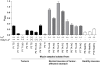
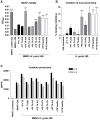
Figure 6. Gene expression of H. pylori in response to mucins.
A–E) The gene expression of cagA, ureA, b_abA_, sabA and flaA of H. pylori J99 wt cultured in the presence of 50 µg/mL mucin samples derived from tumor tissue (black bars), normal mucosa of tumor-affected stomachs (grey; filled bars = surface mucins, striped bars = gland mucins) and healthy mucosa (white bars). Expressions are shown as fold expression to H. pylori cultured without mucins, normalized to 16S rRNA. A fold expression difference greater than 4 is considered significant. F) J99Δ_babA_Δ_sabA_ fold expression of cagA and ureA.
Figure 7. Effects on mucin concentration and host cell adhesion on viability and cytokine production after infection.
A) H. pylori J99 wt proliferation with patient 1 soluble tumor mucins and 50 µg/mL glycoconjugates after 24 h culture in RPMI 10% FBS, prior to MKN7 infection, expressed as fold change from start of culture. B) Binding of H. pylori to MKN7 cells after 24 h infection expressed as percentages less binding than untreated H. pylori. C) Viability of MKN7 cells measured by cell metabolic activity (WST-1 assay) after 24 h infection. Data are shown after subtracting the small signal produced by the presence of bacteria in the assay. D) Percentage of live, early and late apoptotic cells after 24 h infection measured by flow cytometry (Annexin V/7AAD). E) Concentration of IL-6 and IL-8 in cell culture media after 24 h infection (One out of two experiments carried out at different concentrations with similar results are shown). (***p≤0.001, **p<0.01, *p<0.05, ANOVA, Dunnett t-test, compared to J99 without mucins or with HSA control. Independent Student's t-test was used to compare between Leb and sialyl-Lex glycoconjugates.)
Figure 8. Host cell adhesion, viability and cytokine production after infection with H. pylori pretreated with different mucins.
A) Viability of MKN7 cells measured by the cell proliferation reagent WST-1 after 24 h infection with H. pylori pretreated with mucin samples derived from tumor tissue (black bars), normal mucosa of tumor-affected stomachs (grey; filled bars = surface mucins, striped bars = gland mucins) and healthy mucosa (white bars), compared to controls without mucins (black and white bars). B) Binding of H. pylori to MKN7 cells after 24 h infection expressed as percentages less binding than untreated H. pylori. C) Concentration of IL-6 and IL-8 in cell culture media after 24 h infection (One out of two experiments carried out at different concentrations with similar results are shown). (***p≤0.001, **p<0.01, *p<0.05, ANOVA, Dunnett t-test, compared to MKN7+J99 without mucins.)
Figure 9. IL-8 production in response to H. pylori infection is concentration and adhesion dependent.
A) Fold change of IL-8 concentration in cell culture media from MKN7 cells infected with H. pylori J99 wt and J99Δ_babA_Δ_sabA_ (multiplicity of infection (MOI) of 5) compared to uninfected cells. The ratio of host cell binding of J99Δ_babA_Δ_sabA_ to J99 wt is indicated in blue. B) Fold change of IL-8 concentration after infection with H. pylori J99 wt at a MOI of 5 and 1, and with H. pylori J99 wt supernatant, compared to uninfected cells.
Figure 10. Isolation of mucins from gastric tissue.
Panels show carbohydrate, MUC5AC and MUC2 content in density gradient fractions diluted 1∶500. The fractions for all samples were pooled based on this type of graphs, and in these graphs the pooled fractions are indicated by brackets. A) Density gradient of the patient 1 insoluble tumor mucin sample, which is pooled into two samples with most MUC5AC in the high density sample and most MUC2 in the low density sample. B) Density gradient of the patient 6 tumor-adjacent antrum surface sample.
Similar articles
- BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS.
Skoog EC, Padra M, Åberg A, Gideonsson P, Obi I, Quintana-Hayashi MP, Arnqvist A, Lindén SK. Skoog EC, et al. Sci Rep. 2017 Jan 20;7:40656. doi: 10.1038/srep40656. Sci Rep. 2017. PMID: 28106125 Free PMC article. - [The role of gastric mucins in interactions with Helicobacter pylori].
Radziejewska I. Radziejewska I. Postepy Hig Med Dosw (Online). 2012 Jan 30;66:60-6. Postepy Hig Med Dosw (Online). 2012. PMID: 22371407 Review. Polish. - Muc5ac gastric mucin glycosylation is shaped by FUT2 activity and functionally impacts Helicobacter pylori binding.
Magalhães A, Rossez Y, Robbe-Masselot C, Maes E, Gomes J, Shevtsova A, Bugaytsova J, Borén T, Reis CA. Magalhães A, et al. Sci Rep. 2016 May 10;6:25575. doi: 10.1038/srep25575. Sci Rep. 2016. PMID: 27161092 Free PMC article. - Effects of pH on Helicobacter pylori binding to human gastric mucins: identification of binding to non-MUC5AC mucins.
Lindén S, Mahdavi J, Hedenbro J, Borén T, Carlstedt I. Lindén S, et al. Biochem J. 2004 Dec 1;384(Pt 2):263-70. doi: 10.1042/BJ20040402. Biochem J. 2004. PMID: 15260802 Free PMC article. - Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors.
Magalhães A, Reis CA. Magalhães A, et al. Braz J Med Biol Res. 2010 Jul;43(7):611-8. doi: 10.1590/s0100-879x2010007500049. Epub 2010 Jun 7. Braz J Med Biol Res. 2010. PMID: 20521012 Review.
Cited by
- Structural diversity of human gastric mucin glycans.
Jin C, Kenny DT, Skoog EC, Padra M, Adamczyk B, Vitizeva V, Thorell A, Venkatakrishnan V, Lindén SK, Karlsson NG. Jin C, et al. Mol Cell Proteomics. 2017 Mar 13;16(5):743-58. doi: 10.1074/mcp.M117.067983. Online ahead of print. Mol Cell Proteomics. 2017. PMID: 28289177 Free PMC article. - Divergence between the Highly Virulent Zoonotic Pathogen Helicobacter heilmannii and Its Closest Relative, the Low-Virulence "Helicobacter ailurogastricus" sp. nov.
Joosten M, Lindén S, Rossi M, Tay AC, Skoog E, Padra M, Peters F, Perkins T, Vandamme P, Van Nieuwerburgh F, D'Herde K, Van den Broeck W, Flahou B, Deforce D, Ducatelle R, Marshall B, Haesebrouck F, Smet A. Joosten M, et al. Infect Immun. 2015 Nov 2;84(1):293-306. doi: 10.1128/IAI.01300-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26527212 Free PMC article. - Chilean Rhubarb, Gunnera tinctoria (Molina) Mirb. (Gunneraceae): UHPLC-ESI-Orbitrap-MS Profiling of Aqueous Extract and its Anti-Helicobacter pylori Activity.
Hebel-Gerber S, García-Cancino A, Urbina A, Simirgiotis MJ, Echeverría J, Bustamante-Salazar L, Sáez-Carrillo K, Alarcón J, Pastene-Navarrete E. Hebel-Gerber S, et al. Front Pharmacol. 2021 Jan 27;11:583961. doi: 10.3389/fphar.2020.583961. eCollection 2020. Front Pharmacol. 2021. PMID: 33708110 Free PMC article. - Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes.
Padra M, Adamczyk B, Benktander J, Flahou B, Skoog EC, Padra JT, Smet A, Jin C, Ducatelle R, Samuelsson T, Haesebrouck F, Karlsson NG, Teneberg S, Lindén SK. Padra M, et al. Virulence. 2018 Dec 31;9(1):898-918. doi: 10.1080/21505594.2018.1460979. Virulence. 2018. PMID: 29638186 Free PMC article. - Porcine Gastric Mucin Triggers Toxin Production of Enteropathogenic Bacillus cereus.
Jessberger N, Dietrich R, Mohr AK, Da Riol C, Märtlbauer E. Jessberger N, et al. Infect Immun. 2019 Mar 25;87(4):e00765-18. doi: 10.1128/IAI.00765-18. Print 2019 Apr. Infect Immun. 2019. PMID: 30745328 Free PMC article.
References
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources