Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages - PubMed (original) (raw)
. 2012 Jun 4;209(6):1167-81.
doi: 10.1084/jem.20120340. Epub 2012 May 7.
Yilin Wang, Melanie Greter, Peter See, Pearline Teo, Benoit Malleret, Marylène Leboeuf, Donovan Low, Guillaume Oller, Francisca Almeida, Sharon H Y Choy, Marcos Grisotto, Laurent Renia, Simon J Conway, E Richard Stanley, Jerry K Y Chan, Lai Guan Ng, Igor M Samokhvalov, Miriam Merad, Florent Ginhoux
Affiliations
- PMID: 22565823
- PMCID: PMC3371735
- DOI: 10.1084/jem.20120340
Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages
Guillaume Hoeffel et al. J Exp Med. 2012.
Abstract
Langerhans cells (LCs) are the dendritic cells (DCs) of the epidermis, forming one of the first hematopoietic lines of defense against skin pathogens. In contrast to other DCs, LCs arise from hematopoietic precursors that seed the skin before birth. However, the origin of these embryonic precursors remains unclear. Using in vivo lineage tracing, we identify a first wave of yolk sac (YS)-derived primitive myeloid progenitors that seed the skin before the onset of fetal liver hematopoiesis. YS progenitors migrate to the embryo proper, including the prospective skin, where they give rise to LC precursors, and the brain rudiment, where they give rise to microglial cells. However, in contrast to microglia, which remain of YS origin throughout life, YS-derived LC precursors are largely replaced by fetal liver monocytes during late embryogenesis. Consequently, adult LCs derive predominantly from fetal liver monocyte-derived cells with a minor contribution of YS-derived cells. Altogether, we establish that adult LCs have a dual origin, bridging early embryonic and late fetal myeloid development.
Figures
Figure 1.
Adult LCs arise from embryonic precursors. Newborn CD45.2+ mice were reconstituted with bone marrow cells isolated from adult CD45.1+ mice. (A) Percentage donor-derived cell populations 3 mo after newborn transplantation (TX). Each data point represents a single mouse. Bars represent means of data from 4 pooled experiments (n = 11). The gating strategy for each leukocyte population was described previously (Ginhoux et al., 2010). (B) Percentage donor-derived monocytes, microglial cells, and LCs at different time points after reconstitution. Bars represent means of data from 4 pooled experiments (n = 4–11). ***, P < 0.0001; **, P < 0.001; *, P < 0.01.
Figure 2.
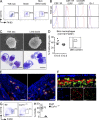
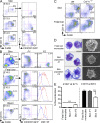
Fetal macrophages are already present in the skin rudiment of E12.5 embryos. (A and B) CD11b and F4/80 expression on gated DAPI−CD45+ cells isolated from indicated tissues of E12.5 embryos. (B) Expression of indicated cell surface markers or GFP reporters (red) compared with isotype/WT controls (blue) on DAPI−CD45+CD11b+F4/80+ cells from YS or skin (limb buds) isolated from WT, Cx3cr1gfp/+, and Csf1rgfp/+ embryos. Data are representative of 3 independent experiments (n = 6 mice). (C) E12.5 YS or limb bud macrophages were sorted based on their phenotype (DAPI−CD45+CD11b+F4/80+) and harvested onto cytospin slides for SEM microscopy (top; bar, 1 µm) or Wright-Giemsa staining (bottom; bar, 5 µm). Data are representative of two independent experiments. (D) Flow cytometric cell cycle analysis of CD45+F4/80+CD11b+ macrophages present in E10.5 and E12.5 limb buds. Data from two pooled experiments (n = 6 to 7). (E) Cross sections of developing skin obtained from E12.5 Cx3cr1gfp/+ embryos were labeled with anti-F4/80 monoclonal antibody (red) and counterstained with DAPI that label nuclei (blue). Data are representative of 2 experiments (n = 2; bar, 30 µm). (F) High magnification image of CSF-1Rgfp cells (green) in Csf1rgfp/+ E12.5 developing skin acquired by two photon microscopy. Evans blue stains the blood vessels (red) and DAPI (blue) stains the superficial layer of the epidermis. Top panel illustrates the side view of these 3D reconstructions, and top view snapshots at different depths are shown in the bottom panels (Bars: I, ∼30 µm; II, ∼60 µm; III, ∼90 µm). Bars: 80 µm (top); 30 µm (side). Data are representative of 2 experiments (n = 2). (G and H) CD11b and F4/80 expression on gated DAPI−CD45+ cells isolated from E12.5 Csf-1r−/− or control littermate (Wt) FVB/NJ embryos. (H) Percentage fetal macrophages (F4/80+CD11b+) isolated from E12.5 Csf-1r−/− or control littermate (Wt) mice. Pooled data from three separate experiments. **, P < 0.001.
Figure 3.
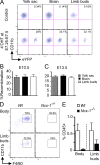
YS-derived primitive macrophages are the major precursors of skin-infiltrating macrophages in E10.5 and E13.5 embryos. (A–C) Runx1Cre/wt : Rosa26R26R-eYFP embryos were treated with 4’OHT to induce Cre-mediated recombination at E7.25–E7.5 followed by analysis at E10.5 (A and B) or E13.5 (C). Controls are nontreated mice. (A) Flow cytometric analysis from one representative control or treated E10.5 embryo, showing the percentage recombination (eYFP+ cells) within CD45+CD11b+F4/80+ cells isolated from YS, brain rudiment, and limb buds at E10.5. (B and C) Percentage recombination within CD45+CD11b+F4/80+ cells isolated from the indicated tissues at E10.5 and E13.5. Error bars represent mean ± SEM of pooled data from 2 experiments (n = 8–16). (D and E) Developing skin (body and limb buds) was isolated from E10.0–E10.5 Ncx1−/− embryos or control littermates and processed for flow cytometric analysis. (E) Percentage ± SEM of hematopoietic cells (CD45+) in control littermates (white bars, n = 4) and Ncx1−/− embryos (black bars, n = 3). Pooled data from two independent experiments.
Figure 4.
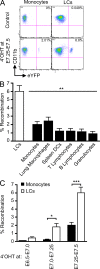
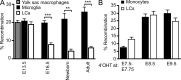
Primitive YS-derived macrophages contribute to adult LCs. Runx1Cre/wt : Rosa26R26R-eYFP embryos were treated with 4’OHT to induce Cre-mediated recombination at different time-points of development and analyzed during adulthood at 8–10 wk post-birth. Controls are nontreated mice. (A) Flow cytometric analysis from one representative control adult or 4’OHT-treated adult (treated at E7.25-E7.5), showing the % recombination (eYFP+ cells) within blood monocytes and epidermal LCs. (B) Pooled data from 3 experiments showing the % recombination among epidermal LCs and various indicated cell population from mice treated with 4’OHT at E7.25-E7.5. (C) Percentage recombination within monocytes (black bars) and epidermal LCs (white bars) in adult mice treated with 4’OHT at the indicated embryonic ages. Error bars represent mean ± SEM of pooled data from 2 experiments (n = 8). ***, P < 0.0001, **, P < 0.001; *, P < 0.01.
Figure 5.
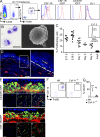
Characterization of LC precursors in embryonic early epidermis. (A) Flow cytometric analysis of gated DAPI−CD45+ cells from E17.5 epidermis. Histograms show expression of cell surface markers or GFP reporters (red) compared with isotype/WT controls (blue) on gated DAPI−CD45+CD11b+F4/80+ cells. Data are representative of 3 independent experiments (n = 6 mice). (B) Cytospin and SEM of sorted E17.5 myeloid cells (as in Fig. 2 C). Data are representative of two independent experiments. (C) Flow cytometric cell cycle analysis of CD45+F4/80+CD11b+ LC precursors at different stages of embryonic development or early postnatal development and adult LCs. Data from 2 pooled experiments (n = 4–13 mice). (D) Skin cross sections obtained from E16.5 Cx3cr1gfp/+ embryos were labeled with anti-F4/80 monoclonal antibody (red) and counterstained with DAPI that label nuclei (blue). Higher magnification image reveals the presence of F4/80+CX3CR1/GFP+ LC precursors in the epidermis at E16.5 (bar, 50 µm). Data are representative of 2 independent experiments (n = 4). (E) Three-dimensional reconstructions of image stacks taken by multiphoton microscopy from E16.5 developing skin of Cx3cr1gfp/+ or Csf1rgfp/+ embryos. DAPI (blue) stains the superficial layer of the epidermis, and blood vessels are highlighted in red using Evans blue (as in Fig. 2 F; data are representative of 2 experiments, n = 2). Bars: 80 µm (top); 30 µm (side). (F and G) Percentage LC precursors (F4/80+CD11b+) among DAPI−CD45+ circulating leukocytes isolated from E17.5 Csf-1r−/− or control littermate (Wt) FVB/NJ embryos. Pooled data from three separate experiments.
Figure 6.
YS macrophage-derived LC precursors are partly replaced during late embryogenesis. (A) Percentage recombination among E13.5 YS macrophages (CD45+CD11b+F4/80+Gr-1−; gray bar); E13.5 and E16.5 microglial progenitors (CD45+CD11b+F4/80+Gr-1−) and adult microglia (CD45loCD11b+F4/80+Gr-1−; black bars); and limb buds YS macrophages (E13.5), epidermal LC precursors (E16.5, newborn), and adult LCs (white bars) from Runx1Cre/wt :Rosa26_R26R-eYFP_ embryos treated with 4’OHT at E7.25-E7.5. Error bars represent mean ± SEM of pooled data from 2 experiments (n = 6–15). **, P < 0.01; ***, P < 0.0001. (B) Percentage recombination within monocytes and epidermal LCs in adult mice treated with 4’OHT at the indicated embryonic ages. Error bars represent mean ± SEM of pooled data from 2 experiments (n = 3–15 mice).
Figure 7.
Fetal liver monocytes are recruited to the developing skin from E14.5. (A and B) Expression of Gr-1 and CX3CR1gfp in gated DAPI−CD45+CD11b+F4/80+ cells isolated from the indicated tissues of Cx3cr1gfp/+ embryos at the indicated stage of development. (B) Histograms show expression of CSF-1R (red) compared with isotype control (blue) on gated cells. Data are representative of 3 independent experiments (n = 3). (C) Expression of CD11b and F4/80 on gated DAPI−CD45+ cells isolated from the skin or fetal liver of E15.5 Csf-1r−/− or control littermate (Wt) FVB/NJ mice. Data are representative of 2 independent experiments (n = 3). (D) Indicated populations were sorted from E16.5 Cx3cr1gfp/+ embryos based from on their described phenotype and harvested onto cytospin slides for Wright-Giemsa staining (left; bar, 5 µm) or SEM microscopy (right; bar, 1 µm). Data are representative of two independent experiments. (E) Percentage recombination within the indicated gated populations isolated from E16.5 fetal liver or prospective dermis from Runx1Cre/wt : Rosa26_R26R-eYFP_ embryos treated with 4’OHT at E7.25–E7.5 or E8.5. Error bars represent mean ± SEM of pooled data from 2 experiments (n = 6–12). ***, P < 0.0001.
Figure 8.
Fetal liver monocytes differentiate into LC precursors. Monocytes were purified from E13.5–E14.5 fetal liver of _Cx3cr1gfp/+_CD45.1+ mice and adoptively transferred in utero into unconditioned E13.5–E14.5 CD45.2+ congenic embryos. (A–C) Flow cytometry analysis of the progeny of adoptively transferred CD45.1+ monocytes (A) in the blood (B) and skin (C) 3 h after injection. (A) Gating strategy (top) for monocytes among fetal liver leukocytes (DAPI−CD45+), purity after sorting (middle), and profile of expression for Gr-1 and CX3CR1/GFP (bottom). (B and C) Percentage CD45.1+ donor-derived monocytes among total CD45+ cells (top), expression of CD11b and F4/80 (middle), and Gr-1 and CX3CR1/GFP (bottom) among indicated populations. (D) Percentage cells derived from donor monocytes for each injected embryo (n = 7) in E14.5 blood and skin 3 h after transfer. Control represents noninjected embryos (n = 9). Bars show means of data from one representative experiment of three. (E) Percentage populations P1 (CD11bhiF480lo) and P2 (CD11bloF480hi) on gated DAPI−CD11b+F480+CD45.1+ donor-derived cells isolated from the skin at indicated time points after transfer (E14.5) and their corresponding profile of expression for Gr-1 and CX3CR1/GFP. (F) Percentage population P2 on gated DAPI−CD11b+F480+CD45.1+ donor derived cells isolated from the skin at indicated time points after transfer. Error bars represent mean ± SEM of pooled data from two experiments (n = 3 to 8). (G) Graph shows the percentage of skin CD11b+F4/80+ cells derived from donor fetal liver monocytes (white squares) or donor fetal liver Ter119+ erythroid progenitors (gray squares; n = 4–11) for each injected embryo at the indicated time points. Bars represent means of data at each time point. ***, P < 0.0001; *, P < 0.01.
Figure 9.
Fetal liver monocytes contribute to epidermal LCs. Monocytes were purified from E13.5–E14.5 fetal liver of CD45.1+ mice and adoptively transferred in utero into unconditioned E13.5–E14.5 CD45.2+ congenic embryos. (A) Flow cytometry analysis of the progeny of adoptively transferred CD45.1+ monocytes in E19.5 epidermis 5 d after transfer. Control represents noninjected embryos (n = 9). (B) Percentage cells derived from donor monocytes for each injected embryos in the epidermis 5 d after injection. Bars show means of data from one representative experiment of three. (C) Expression of MHC-II on donor CD45.1+ monocyte-derived LC precursors at the indicated time points. Data are representative of 2 independent experiments (n = 2). (D) Percentage E18.5 donor (CD45.1+) LC precursors (CD11b+F480+CX3CR1/GFPhi) derived from adoptively transferred Gr-1hi or Gr-1lo fetal liver monocytes (5 × 105 per embryo). Error bars represent mean ± SEM of pooled data from 2 experiments (n = 2–5).
Similar articles
- Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors.
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Gomez Perdiguero E, et al. Nature. 2015 Feb 26;518(7540):547-51. doi: 10.1038/nature13989. Epub 2014 Dec 3. Nature. 2015. PMID: 25470051 Free PMC article. - C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages.
Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. Hoeffel G, et al. Immunity. 2015 Apr 21;42(4):665-78. doi: 10.1016/j.immuni.2015.03.011. Immunity. 2015. PMID: 25902481 Free PMC article. - Fetal monocytes and the origins of tissue-resident macrophages.
Hoeffel G, Ginhoux F. Hoeffel G, et al. Cell Immunol. 2018 Aug;330:5-15. doi: 10.1016/j.cellimm.2018.01.001. Epub 2018 Jan 12. Cell Immunol. 2018. PMID: 29475558 Review. - Embryonic hematopoiesis.
Golub R, Cumano A. Golub R, et al. Blood Cells Mol Dis. 2013 Dec;51(4):226-31. doi: 10.1016/j.bcmd.2013.08.004. Epub 2013 Sep 13. Blood Cells Mol Dis. 2013. PMID: 24041595 Review. - A lineage of myeloid cells independent of Myb and hematopoietic stem cells.
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. Schulz C, et al. Science. 2012 Apr 6;336(6077):86-90. doi: 10.1126/science.1219179. Epub 2012 Mar 22. Science. 2012. PMID: 22442384
Cited by
- Epidermal maintenance of Langerhans cells relies on autophagy-regulated lipid metabolism.
Arbogast F, Sal-Carro R, Boufenghour W, Frenger Q, Bouis D, Filippi De La Palavesa L, Fauny JD, Griso O, Puccio H, Fima R, Huby T, Gautier EL, Molitor A, Carapito R, Bahram S, Romani N, Clausen BE, Voisin B, Mueller CG, Gros F, Flacher V. Arbogast F, et al. J Cell Biol. 2025 Feb 3;224(2):e202403178. doi: 10.1083/jcb.202403178. Epub 2024 Nov 13. J Cell Biol. 2025. PMID: 39535446 Free PMC article. - Adipose Tissue Macrophages of the Human Fetus.
Radványi Á, Gyurina K, Rácz E, Kovács I, Méhes G, Röszer T. Radványi Á, et al. Cells. 2024 Oct 28;13(21):1787. doi: 10.3390/cells13211787. Cells. 2024. PMID: 39513894 Free PMC article. - When Histiocytosis Masquerades as Mononucleosis: A Case Report.
Khundadze M, Khurtsia L, Shulaia N, Kandelaki G. Khundadze M, et al. Cureus. 2024 Sep 25;16(9):e70156. doi: 10.7759/cureus.70156. eCollection 2024 Sep. Cureus. 2024. PMID: 39463558 Free PMC article. - Macrophage variants in laboratory research: most are well done, but some are RAW.
Herb M, Schatz V, Hadrian K, Hos D, Holoborodko B, Jantsch J, Brigo N. Herb M, et al. Front Cell Infect Microbiol. 2024 Oct 9;14:1457323. doi: 10.3389/fcimb.2024.1457323. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39445217 Free PMC article. Review. - Induced pluripotent stem cell-derived macrophages as a platform for modelling human disease.
Tiwari SK, Wong WJ, Moreira M, Pasqualini C, Ginhoux F. Tiwari SK, et al. Nat Rev Immunol. 2024 Sep 27. doi: 10.1038/s41577-024-01081-x. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39333753 Review.
References
- Bogunovic M., Ginhoux F., Wagers A., Loubeau M., Isola L.M., Lubrano L., Najfeld V., Phelps R.G., Grosskreutz C., Scigliano E., et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 203:2627–2638 10.1084/jem.20060667 - DOI - PMC - PubMed
- Breathnach A.S., Silvers W.K., Smith J., Heyner S. 1968. Langerhans cells in mouse skin experimentally deprived of its neural crest component. J. Invest. Dermatol. 50:147–160 - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI080884/AI/NIAID NIH HHS/United States
- AI080884/AI/NIAID NIH HHS/United States
- R01 HL086899/HL/NHLBI NIH HHS/United States
- R37 CA026504/CA/NCI NIH HHS/United States
- HL086899/HL/NHLBI NIH HHS/United States
- CA112100/CA/NCI NIH HHS/United States
- R01 CA112100/CA/NCI NIH HHS/United States
- U01 AI095611/AI/NIAID NIH HHS/United States
- R01 CA026504/CA/NCI NIH HHS/United States
- R01 CA032551/CA/NCI NIH HHS/United States
- R01 CA154947/CA/NCI NIH HHS/United States
- CA32551/CA/NCI NIH HHS/United States
- CA26504/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases