The role of heat shock proteins in antigen cross presentation - PubMed (original) (raw)
The role of heat shock proteins in antigen cross presentation
Ayesha Murshid et al. Front Immunol. 2012.
Abstract
Heat shock proteins (HSPs) are molecular chaperones that bind tumor antigens and mediate their uptake into antigen presenting cells. HSP-antigen complexes are then directed toward either the MHC class I pathway through antigen cross presentation or the conventional class II pathway, leading to activation of T cell subsets. Uptake of HSP-chaperoned polypeptides can involve both receptor-mediated and receptor-independent routes, and mechanisms of antigen sorting between the Class I and II pathways after uptake are currently under investigation. The processes involved in internalization of HSP-antigen complexes differ somewhat from the mechanisms previously determined for (unchaperoned) particulate and free soluble antigens. A number of studies show that HSP-facilitated antigen cross presentation requires uptake of the complexes by scavenger receptors (SR) followed by processing in the proteasome, and loading onto MHC class I molecules. In this review we have examined the roles of HSPs and SR in antigen uptake, sorting, processing, cell signaling, and activation of innate and adaptive immunity.
Keywords: CTL response; anti-cancer vaccine; antigen cross presentation; antigen presenting cells; heat shock proteins; scavenger receptor; soluble vs. particulate antigen; tumor immunity.
Figures
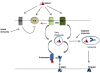
Figure 1
Antigen presentation pathways for HSP-bound polypeptides. HSP.PCs bind to surface receptors on DC including SRECI and LOX-1 and are internalized in complexes with the receptors. Binding to these receptors may also trigger secondary activation of Toll-like receptors (TLR2 or TLR4) that may amplify antigen cross presentation. Alternatively some studies suggest direct binding of HSPs to TLR. HSP binding to cell surface SRA/CD204 is inhibitory to TLR4 activity and likely antagonizes the immune responses induced by HSPs through LOX-1 or SRECI. HSP.PC are internalized by SRECI or LOX-1 into endosomes with the subsequent release of the peptides from the HSP.PC complex. Such peptides are then processed either within endosomes or undergo trafficking to the cytosol where they encounter the proteasomal system. Processed peptides are then loaded either onto MHC class I in the ER or phagosomes or onto MHC class II molecules in lysosomes and presented to, respectively CD8+ T cells and CD4+ T cells. The triage mechanisms directing HSP-chaperoned peptides toward either of these two pathways are currently not known.
Similar articles
- Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells.
Tobian AA, Canaday DH, Harding CV. Tobian AA, et al. J Immunol. 2004 Oct 15;173(8):5130-7. doi: 10.4049/jimmunol.173.8.5130. J Immunol. 2004. PMID: 15470057 - Immunological Outcomes Mediated Upon Binding of Heat Shock Proteins to Scavenger Receptors SCARF1 and LOX-1, and Endocytosis by Mononuclear Phagocytes.
Murshid A, Borges TJ, Bonorino C, Lang BJ, Calderwood SK. Murshid A, et al. Front Immunol. 2020 Jan 10;10:3035. doi: 10.3389/fimmu.2019.03035. eCollection 2019. Front Immunol. 2020. PMID: 31998315 Free PMC article. Review. - Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways.
Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Castellino F, et al. J Exp Med. 2000 Jun 5;191(11):1957-64. doi: 10.1084/jem.191.11.1957. J Exp Med. 2000. PMID: 10839810 Free PMC article. - Immunostimulatory functions of membrane-bound and exported heat shock protein 70.
Radons J, Multhoff G. Radons J, et al. Exerc Immunol Rev. 2005;11:17-33. Exerc Immunol Rev. 2005. PMID: 16385841 Review.
Cited by
- Upregulation of PD-1/PD-L1 and downregulation of immune signaling pathways lead to more severe visceral leishmaniasis in undernutrition mice.
He J, Zhang J, Liao X, Xiao Y, Li J, Zheng Z, Chen D, Chen J. He J, et al. Parasit Vectors. 2024 Jan 8;17(1):8. doi: 10.1186/s13071-023-06018-2. Parasit Vectors. 2024. PMID: 38185681 Free PMC article. - Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma.
Shevtsov MA, Yakovleva LY, Nikolaev BP, Marchenko YY, Dobrodumov AV, Onokhin KV, Onokhina YS, Selkov SA, Mikhrina AL, Guzhova IV, Martynova MG, Bystrova OA, Ischenko AM, Margulis BA. Shevtsov MA, et al. Neuro Oncol. 2014 Jan;16(1):38-49. doi: 10.1093/neuonc/not141. Epub 2013 Dec 4. Neuro Oncol. 2014. PMID: 24305705 Free PMC article. - Heat shock protein 70 protects the lungs from hyperoxic injury in a neonatal rat model of bronchopulmonary dysplasia.
Lee CH, Su TC, Lee MS, Hsu CS, Yang RC, Kao JK. Lee CH, et al. PLoS One. 2023 May 18;18(5):e0285944. doi: 10.1371/journal.pone.0285944. eCollection 2023. PLoS One. 2023. PMID: 37200358 Free PMC article. - High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy.
Wang XY, Subjeck JR. Wang XY, et al. Int J Hyperthermia. 2013 Aug;29(5):364-75. doi: 10.3109/02656736.2013.803607. Epub 2013 Jul 5. Int J Hyperthermia. 2013. PMID: 23829534 Free PMC article. Review. - Heat Shock Proteins: Central Players in Oncological and Immuno-Oncological Tracks.
Youness RA, Gohar A, Kiriacos CJ, El-Shazly M. Youness RA, et al. Adv Exp Med Biol. 2023;1409:193-203. doi: 10.1007/5584_2022_736. Adv Exp Med Biol. 2023. PMID: 36038808
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials