Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation - PubMed (original) (raw)
Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation
Narender R Gavva et al. Mol Pain. 2012.
Abstract
Background: Transient receptor potential cation channel subfamily M member 8 (TRPM8) is activated by cold temperature in vitro and has been demonstrated to act as a 'cold temperature sensor' in vivo. Although it is known that agonists of this 'cold temperature sensor', such as menthol and icilin, cause a transient increase in body temperature (Tb), it is not known if TRPM8 plays a role in Tb regulation. Since TRPM8 has been considered as a potential target for chronic pain therapeutics, we have investigated the role of TRPM8 in Tb regulation.
Results: We characterized five chemically distinct compounds (AMG0635, AMG2850, AMG8788, AMG9678, and Compound 496) as potent and selective antagonists of TRPM8 and tested their effects on Tb in rats and mice implanted with radiotelemetry probes. All five antagonists used in the study caused a transient decrease in Tb (maximum decrease of 0.98°C). Since thermoregulation is a homeostatic process that maintains Tb about 37°C, we further evaluated whether repeated administration of an antagonist attenuated the decrease in Tb. Indeed, repeated daily administration of AMG9678 for four consecutive days showed a reduction in the magnitude of the Tb decrease Day 2 onwards.
Conclusions: The data reported here demonstrate that TRPM8 channels play a role in Tb regulation. Further, a reduction of magnitude in Tb decrease after repeated dosing of an antagonist suggests that TRPM8's role in Tb maintenance may not pose an issue for developing TRPM8 antagonists as therapeutics.
Figures
Figure 1
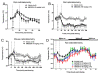
Characterization of five distinct compounds as TRPM8 antagonists. A) chemical structures of antagonists used in the study. B) Concentration dependent effects of antagonists on menthol-induced intracellular calcium increase in CHO cells stably expressing rat TRPM8. C) Concentration dependent effects of antagonists on cold (10°C)-induced intracellular calcium increase in CHO cells stably expressing rat TRPM8. Each data point in the graph are average ± S.D. of an experiment conducted in triplicate.
Figure 2
Effects of TRPM8 antagonists on body temperature (T b ) in rats or mice. Data are presented as mean ± S.E.M. of temperature collected for every 10 min. Statistical significance is relative to the vehicle (one tail unpaired _t_-test). Baseline Tb was collected for 20–30 min before compound administration (p.o.) at time 0 and post dosing every 10 min for 120 min (A) or 240 min ( B &C). The stress-induced transient increase in Tb seen right after antagonist administration is indicated by vertical dotted lines. A) AMG8788 dosed at 30 mg/kg significantly decreased rat Tb by 0.53°C at 40 min (_t_10 = 2.55; p < 0.05). B) AMG2850 dosed at 100 mg/kg significantly decreased rat Tb by 0.98°C at 140 min (_t_10 = 4.38; p < 0.001). C) AMG2850 dosed at 100 mg/kg significantly decreased mouse Tb by 0.73°C at 100 min (_t_17 = 2.99; p < 0.001). D) TRPM8 antagonist AMG9678 induced decrease in body temperature is transient in nature. Statistical significance is relative to vehicle (one way ANOVA followed by Dunnett’s Multiple Comparison Test). Baseline Tb was collected at 30 min before compound administration (p.o.) at time 0 and post dosing every 1 h for 24 h. At 100 mg/kg, AMG9678 significantly decreased Tb by 0.83°C at 1 hour (_F_3,22 = 6.46, p < 0.01), whereas at the same time, the maximum decrease of Tb was 0.7 and 0.72 0 C at 30 mg/kg and 10 mg/kg, respectively.
Figure 3
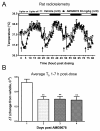
AMG9678-induced decrease in T b partially attenuates after repeat dosing in rats. AMG9678 was administered (p.o.) each day at 9:00 am for 4 days as indicated by an arrow. Tb data was collected every hour for 80 h and are presented as mean ± S.E.M. A) AMG9678 at 30 mg/kg produced a significant decrease of Tb by 0.62°C at 5 h (_t_14 = 4.27, p < 0.001), 0.47°C at 26 h ( _t_14 = 4.95, p < 0.001), 0.51°C at 52 h ( _t_14 = 5.01, p < 0.0001), and 0.38°C at 75 h ( _t_14 = 2.68, p < 0.01), respectively. The decrease in Tb lasted for 7 h post 1st dosing, 5 h post 2nd dosing, 5 h post 3rd dosing and 6 h post 4th dosing, respectively. B. AMG9678-induced decrease in Tb reduced on day 2–4 compared to day 1. Bars represent mean ± S.E.M. of ΔT of average 1–7 h post dosing on days 1–4.
Similar articles
- AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia.
Lehto SG, Weyer AD, Zhang M, Youngblood BD, Wang J, Wang W, Kerstein PC, Davis C, Wild KD, Stucky CL, Gavva NR. Lehto SG, et al. Naunyn Schmiedebergs Arch Pharmacol. 2015 Apr;388(4):465-76. doi: 10.1007/s00210-015-1090-9. Epub 2015 Feb 10. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25662185 Free PMC article. - Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents.
de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR, Pinter E, Gavva NR, Romanovsky AA. de Oliveira C, et al. J Neurosci. 2014 Mar 26;34(13):4445-52. doi: 10.1523/JNEUROSCI.5387-13.2014. J Neurosci. 2014. PMID: 24671991 Free PMC article. - Recent Progress in TRPM8 Modulation: An Update.
González-Muñiz R, Bonache MA, Martín-Escura C, Gómez-Monterrey I. González-Muñiz R, et al. Int J Mol Sci. 2019 May 28;20(11):2618. doi: 10.3390/ijms20112618. Int J Mol Sci. 2019. PMID: 31141957 Free PMC article. Review. - Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers.
Zakharian E, Cao C, Rohacs T. Zakharian E, et al. J Neurosci. 2010 Sep 15;30(37):12526-34. doi: 10.1523/JNEUROSCI.3189-10.2010. J Neurosci. 2010. PMID: 20844147 Free PMC article. - TRPM8 biology and medicinal chemistry.
DeFalco J, Duncton MA, Emerling D. DeFalco J, et al. Curr Top Med Chem. 2011;11(17):2237-52. doi: 10.2174/156802611796904933. Curr Top Med Chem. 2011. PMID: 21671871 Review.
Cited by
- Discovery of a Selective TRPM8 Antagonist with Clinical Efficacy in Cold-Related Pain.
Andrews MD, Af Forselles K, Beaumont K, Galan SR, Glossop PA, Grenie M, Jessiman A, Kenyon AS, Lunn G, Maw G, Owen RM, Pryde DC, Roberts D, Tran TD. Andrews MD, et al. ACS Med Chem Lett. 2015 Jan 30;6(4):419-24. doi: 10.1021/ml500479v. eCollection 2015 Apr 9. ACS Med Chem Lett. 2015. PMID: 25893043 Free PMC article. - Use of a menthol popsicle in managing postoperative thirst in patients undergoing radical prostatectomy: A randomized clinical trial.
da Silva TTM, Teixeira FC, Matias de Araújo SC, Pinheiro TBM, Fernandes Costa IK, de Medeiros KS, Ribeiro KRB, Dantas DV, Dantas RAN. da Silva TTM, et al. SAGE Open Med. 2023 Oct 13;11:20503121231202231. doi: 10.1177/20503121231202231. eCollection 2023. SAGE Open Med. 2023. PMID: 37846371 Free PMC article. - Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition.
Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Feketa VV, et al. Am J Physiol Regul Integr Comp Physiol. 2013 Nov 1;305(9):R1040-50. doi: 10.1152/ajpregu.00296.2013. Epub 2013 Sep 4. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 24005250 Free PMC article. - Role of TRP channels in the induction of heat shock proteins (Hsps) by heating skin.
Hsu WL, Yoshioka T. Hsu WL, et al. Biophysics (Nagoya-shi). 2015 Feb 13;11:25-32. doi: 10.2142/biophysics.11.25. eCollection 2015. Biophysics (Nagoya-shi). 2015. PMID: 27493511 Free PMC article. Review. - TRPM8 and Migraine.
Dussor G, Cao YQ. Dussor G, et al. Headache. 2016 Oct;56(9):1406-1417. doi: 10.1111/head.12948. Epub 2016 Sep 16. Headache. 2016. PMID: 27634619 Free PMC article. Review.
References
- Hensel H, Zotterman Y. Action potentials of cold fibres and intracutaneous temperature gradient. J Neurophysiol. 1951;14(5):377–85. - PubMed
- Hensel H, Zotterman Y. The response of the cold receptors to constant cooling. Acta Physiol Scand. 1951;22(2–3):96–105. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources