Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women - PubMed (original) (raw)
Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women
Kotha Subbaramaiah et al. Cancer Discov. 2012 Apr.
Retraction in
- Retraction: Increased Levels of COX-2 and Prostaglandin E2 Contribute to Elevated Aromatase Expression in Inflamed Breast Tissue of Obese Women.
Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Subbaramaiah K, et al. Cancer Discov. 2021 May;11(5):1306. doi: 10.1158/2159-8290.CD-21-0224. Cancer Discov. 2021. PMID: 33947719 Free PMC article. No abstract available.
Expression of concern in
- Editor's Note: Increased Levels of COX-2 and Prostaglandin E2 Contribute to Elevated Aromatase Expression in Inflamed Breast Tissue of Obese Women.
[No authors listed] [No authors listed] Cancer Discov. 2019 Aug;9(8):1142. doi: 10.1158/2159-8290.CD-19-0724. Cancer Discov. 2019. PMID: 31371325 No abstract available.
Abstract
Obesity is a risk factor for hormone receptor-positive breast cancer in postmenopausal women. Estrogen synthesis is catalyzed by aromatase, which is encoded by CYP19. We previously showed that aromatase expression and activity are increased in the breast tissue of overweight and obese women in the presence of characteristic inflammatory foci [crown-like structures of the breast (CLS-B)]. In preclinical studies, proinflammatory prostaglandin E(2) (PGE(2)) is a determinant of aromatase expression. We provide evidence that cyclooxygenase (COX)-2-derived PGE(2) stimulates the cyclic AMP (cAMP) → PKA signal transduction pathway that activates CYP19 transcription, resulting in increased aromatase expression and elevated progesterone receptor levels in breast tissues from overweight and obese women. We further demonstrate that a measure of in-breast inflammation (CLS-B index) is a better correlate of these biologic end points than body mass index. The obesity → inflammation → aromatase axis is likely to contribute to the increased risk of hormone receptor-positive breast cancer and the worse prognosis of obese patients with breast cancer.
Significance: We show that obesity-associated inflammatory foci in the human breast are associated with elevated COX-2 levels and activation of the PGE2 → cAMP → PKA signal transduction pathway resulting in increased aromatase expression. These findings help to explain the link among obesity, low-grade chronic inflammation, and breast cancer with important clinical implications.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
A.J. Dannenberg is a member of the Scientific Advisory Board of Tragara Pharmaceuticals, Inc., a company that is developing a selective COX-2 inhibitor. The other authors disclosed no potential conflicts of interest.
Figures
Figure 1
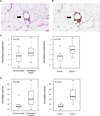
Increased levels of aromatase are found in inflamed breast tissue. Crown-like structure of breast (CLS-B) is shown in panels A and B. A, H&E demonstrating an inflammatory focus containing macrophages surrounding an adipocyte (200×). B, Immunohistochemical stain of CD68 showing the same lesion (200×). In A and B, arrows indicate CLS-B. Levels of aromatase mRNA (C) and activity (D) are increased in inflamed breast tissue (CLS-B + vs. CLS-B −). Box plots are shown for subjects (n=30) varying in weight and breast inflammation status.
Figure 2
Increased levels of COX-2 mRNA, COX-2 protein and PGE2 are found in inflamed breast tissue. Levels of COX-2 mRNA (A), COX-2 protein (B) and PGE2 (C) were compared in breast tissue of normal weight vs. overweight/obese women and in breast tissue with or without evidence of inflammation (CLS-B + vs. CLS-B −). A, quantitative real-time PCR was used to determine COX-2 expression. B, immunoprecipitates were subjected to western blot analysis. Representative results (top of panel) for COX-2 and β-actin are shown vs. the CLS-B index for 17 cases. Densitometry (bottom of panel) was used to quantify COX-2 protein. C, PGE2 was quantified by enzyme immunoassay. A-C, Box plots are shown for subjects (n=30) varying in weight and breast inflammation status.
Figure 3
Increased levels of cAMP and PKA activity are found in inflamed breast tissue. Levels of cAMP (A) and PKA activity (B) were compared in breast tissue of normal weight vs. overweight/obese women and in breast tissue with or without evidence of inflammation (CLS-B + vs. CLS-B −). Box plots are shown for subjects (n=30) varying in weight and breast inflammation status.
Figure 4
Levels of aromatase transcripts derived from promoters I.3 and II and progesterone receptor (PR) are increased in association with breast inflammation. Expression levels of promoter I.3 (A) and promoter II (B) specific aromatase transcripts were quantified by real-time PCR and compared in breast tissue of normal weight vs. overweight/obese women and in breast tissue with or without evidence of inflammation (CLS-B + vs. CLS-B −). C, Immunoprecipitates were subjected to western blot analysis. Representative results (top of panel) for PR and β-actin are shown vs. the CLS-B index for 16 cases. Densitometry (bottom of panel) was used to quantify PR protein. A-C, Box plots are shown for subjects (n=30) varying in weight and breast inflammation status.
Figure 5
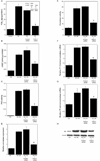
PGE2 derived from stearic acid (SA)-activated macrophages stimulates the cAMP→PKA pathway leading to increased aromatase transcription in preadipocytes. A, THP-1 cells were untreated or treated with control siRNA or siRNA to COX-2. Subsequently, the THP-1 cells were treated with vehicle (Control) or 10 µM SA for 24 hours. The abundance of COX-2 protein in cell lysates (inset) was determined by immunoblotting. β-actin was used as a loading control. Levels of PGE2 in the medium were determined by enzyme immunoassay. B-H, preadipocytes were treated with THP-1 cell-derived CM for 24 hours prior to measurements of cAMP (B), PKA activity (C), relative aromatase expression (D), aromatase activity expressed as femtomoles/µg protein/minute (E), aromatase mRNA derived from promoters I.3 (F) and II (G), and PR protein levels (H). Columns, means (n=6); bars, SD. *, P < 0.05.
Comment in
- The role of the PGE2-aromatase pathway in obesity-associated breast inflammation.
Wang D, DuBois RN. Wang D, et al. Cancer Discov. 2012 Apr;2(4):308-10. doi: 10.1158/2159-8290.CD-12-0078. Cancer Discov. 2012. PMID: 22576207 - Findings of Research Misconduct.
[No authors listed] [No authors listed] Fed Regist. 2023 Sep 13;88(176):62800-62803. Fed Regist. 2023. PMID: 37736072 Free PMC article. No abstract available. - Findings of Research Misconduct.
[No authors listed] [No authors listed] Fed Regist. 2023 Sep 13;88(176):62803-62807. Fed Regist. 2023. PMID: 37736073 Free PMC article. No abstract available.
Similar articles
- Pioglitazone, a PPARγ agonist, suppresses CYP19 transcription: evidence for involvement of 15-hydroxyprostaglandin dehydrogenase and BRCA1.
Subbaramaiah K, Howe LR, Zhou XK, Yang P, Hudis CA, Kopelovich L, Dannenberg AJ. Subbaramaiah K, et al. Cancer Prev Res (Phila). 2012 Oct;5(10):1183-94. doi: 10.1158/1940-6207.CAPR-12-0201. Epub 2012 Jul 10. Cancer Prev Res (Phila). 2012. PMID: 22787115 Free PMC article. Retracted. - The prostaglandin transporter regulates adipogenesis and aromatase transcription.
Subbaramaiah K, Hudis CA, Dannenberg AJ. Subbaramaiah K, et al. Cancer Prev Res (Phila). 2011 Feb;4(2):194-206. doi: 10.1158/1940-6207.CAPR-10-0367. Epub 2011 Jan 6. Cancer Prev Res (Phila). 2011. PMID: 21212407 Retracted. - The role of the PGE2-aromatase pathway in obesity-associated breast inflammation.
Wang D, DuBois RN. Wang D, et al. Cancer Discov. 2012 Apr;2(4):308-10. doi: 10.1158/2159-8290.CD-12-0078. Cancer Discov. 2012. PMID: 22576207 - Interrelationships between cyclooxygenases and aromatase: unraveling the relevance of cyclooxygenase inhibitors in breast cancer.
Díaz-Cruz ES, Brueggemeier RW. Díaz-Cruz ES, et al. Anticancer Agents Med Chem. 2006 May;6(3):221-32. doi: 10.2174/187152006776930873. Anticancer Agents Med Chem. 2006. PMID: 16712450 Review. - Relationship between aromatase and cyclooxygenases in breast cancer: potential for new therapeutic approaches.
Brueggemeier RW, Díaz-Cruz ES. Brueggemeier RW, et al. Minerva Endocrinol. 2006 Mar;31(1):13-26. Minerva Endocrinol. 2006. PMID: 16498361 Review.
Cited by
- Lipid Signaling in Tumorigenesis.
Liu R, Huang Y. Liu R, et al. Mol Cell Pharmacol. 2014 Jan 1;6(1):1-9. Mol Cell Pharmacol. 2014. PMID: 25741396 Free PMC article. - The obesity-inflammation-eicosanoid axis in breast cancer.
Vona-Davis L, Rose DP. Vona-Davis L, et al. J Mammary Gland Biol Neoplasia. 2013 Dec;18(3-4):291-307. doi: 10.1007/s10911-013-9299-z. Epub 2013 Oct 30. J Mammary Gland Biol Neoplasia. 2013. PMID: 24170420 Review. - Omega-3 fatty acids for the prevention of breast cancer: an update and state of the science.
Iyengar NM, Hudis CA, Gucalp A. Iyengar NM, et al. Curr Breast Cancer Rep. 2013 Sep 1;5(3):247-254. doi: 10.1007/s12609-013-0112-1. Curr Breast Cancer Rep. 2013. PMID: 24073296 Free PMC article. - Effects of Neonicotinoid Pesticides on Promoter-Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF Pathway.
Caron-Beaudoin É, Viau R, Sanderson JT. Caron-Beaudoin É, et al. Environ Health Perspect. 2018 Apr 26;126(4):047014. doi: 10.1289/EHP2698. Environ Health Perspect. 2018. PMID: 29701941 Free PMC article. - Relationships Among Obesity, Type 2 Diabetes, and Plasma Cytokines in African American Women.
Denis GV, Sebastiani P, Andrieu G, Tran AH, Strissel KJ, Lombardi FL, Palmer JR. Denis GV, et al. Obesity (Silver Spring). 2017 Nov;25(11):1916-1920. doi: 10.1002/oby.21943. Epub 2017 Aug 25. Obesity (Silver Spring). 2017. PMID: 28840653 Free PMC article.
References
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. - PubMed
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. - PubMed
- Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. Int J Cancer. 2006;118:1915–1921. - PubMed
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials