Leukemia inhibitory factor signaling is required for lung protection during pneumonia - PubMed (original) (raw)
Leukemia inhibitory factor signaling is required for lung protection during pneumonia
Lee J Quinton et al. J Immunol. 2012.
Abstract
Lung infections represent a tremendous disease burden and a leading cause of acute lung injury. STAT3 signaling is essential for controlling lung injury during pneumonia. We previously identified LIF as a prominent STAT3-activating cytokine expressed in the airspaces of pneumonic lungs, but its physiological significance in this setting has never been explored. To do so, Escherichia coli was intratracheally instilled into C57BL/6 mice in the presence of neutralizing anti-LIF IgG or control IgG. Anti-LIF completely eliminated lung LIF detection and markedly exacerbated lung injury compared with control mice as evidenced by airspace albumin content, lung liquid accumulation, and histological analysis. Although lung bacteriology was equivalent between groups, bacteremia was more prevalent with anti-LIF treatment, suggestive of compromised barrier function rather than impaired antibacterial defense as the cause of dissemination. Inflammatory cytokine expression was also exaggerated in anti-LIF-treated lungs, albeit after injury had ensued. Interestingly, alveolar neutrophil recruitment was modestly but significantly reduced compared with control mice despite elevated cytokine levels, indicating that inflammatory injury was not a consequence of excessive neutrophilic alveolitis. Lastly, the lung transcriptome was dramatically remodeled during pneumonia, but far more so following LIF neutralization, with gene changes implicating cell death and epithelial homeostasis among other processes relevant to tissue injury. From these findings, we conclude that endogenous LIF facilitates tissue protection during pneumonia. The LIF-STAT3 axis is identified in this study as a critical determinant of lung injury with clinical implications for pneumonia patients.
Figures
Figure 1
LIF expression and biological activity in the lungs during pneumonia. (A) LIF protein concentrations were quantified in bronchoalveolar lavage fluid (BALF) 0–24 hrs after intratracheal (i.t.) instillation of Escherichia coli. Values are expressed as means ± SEM (n = 4–5). * p < 0.05 compared to uninfected (0h) controls. (B) Immunohistochemistry was used to visualize Y705-phosphorylated STAT3 in histological lung sections prepared from mice treated for 1h with or without i.t. recombinant murine LIF (rmLIF). Background staining was undetectable on sections from i.t. rmLIF mice exposed to an isotype control antibody (not shown). Representative images are shown at 10× and 20× magnification. (C) LIF protein concentrations were quantified in BALF 24 hrs after i.t. E. coli co-instilled with anti-LIF or control IgG. Values are expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 4–5). (D) Y705- phosphorylated STAT3 immunoreactivity was measured by immunoblot in lung homogenates collected from mice 1h after i.t. rmLIF in the presence of 0–10 µg anti-LIF.
Figure 2
Effect of LIF neutralization on acute lung injury. Lungs were collected from mice 24 hrs after intratracheal inoculation of Escherichia coli co-instilled with anti-LIF or control IgG. (A) Representative images are shown for intact freshly isolated lungs and hematoxylin/eosin-stained lung sections. Red circles are used to denote infected left lung lobes. Bronchoalveolar lavage fluid concentrations of (B) mouse albumin and (C) receptor for advanced glycation end products (RAGE), as well as (D) lung wet:dry weight ratios were determined and expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 3–5).
Figure 3
Effect of LIF neutralization on bacterial clearance. Escherichia coli colony-forming units (CFU) were enumerated in blood and lungs after 24 hrs of pneumonia in the presence of anti-LIF or control IgG. CFU/ml (blood) and total lung CFU are shown for individual mice with horizontal lines indicating the median value within each experimental group. * p < 0.05 compared to mice treated with control IgG (n = 9–13).
Figure 4
Effect of LIF neutralization on innate immunity. (A–B) Neutrophil numbers and (C–D) cytokine protein concentrations were quantified in bronchoalveolar lavage fluid (BALF) harvested from mouse lungs 24 or 30 hrs after intratracheal Escherichia coli. Values are expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 4–5).
Figure 5
Effect of LIF supplementation on IL-6 induction in vitro. IL-6 mRNA induction was determined in alveolar macrophages (AM), RAW 264.7 cells and MLE-12 cells following 2 hrs of LPS stimulation in the absence and presence of recombinant murine LIF (rmLIF). Data are expressed as fold-induction compared to cells not treated with LPS. Means ± SEM were calculated by combining data from 3 separate experiments or 3 individual mice (n = 3).
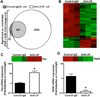
Figure 6
Effects of pneumonia and LIF blockade on the lung transcriptome and markers of cell death. (A–B) Microarrays were performed on total lung RNA 24 hrs after intratracheal (i.t.) instillations of the following 3 combinations: 1) saline and control IgG; 2) Escherichia coli and control IgG; and 3) Escherichia coli and anti-LIF (n = 3). (A) Values indicate the number of differentially expressed genes in each group compared to uninfected mice treated with control IgG alone. (B) A heat map illustrates the expression profile of the 1313 genes significantly different between pneumonic mice treated with control IgG (left 3 columns) or anti-LIF (right 3 columns). For each row (gene), green and red annotations denote decreased or increased expression, respectively. Data in panels (A) and (B) include significantly altered transcripts (FDR < 0.05) with an expression difference of at least 2 fold. qRT-PCR was used to interrogate mRNA fold-induction (compared to control IgG-treated mice) for (C) Fas and (D) VEGF, both of which were significantly affected by anti-LIF treatment as determined by microarray. Individual heat map data are shown for both transcripts to illustrate relative expression levels determined by microarray analysis. * p < 0.05 compared to mice treated with control IgG (n = 3).
Similar articles
- Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia.
Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Quinton LJ, et al. Am J Respir Cell Mol Biol. 2008 Jun;38(6):699-706. doi: 10.1165/rcmb.2007-0365OC. Epub 2008 Jan 10. Am J Respir Cell Mol Biol. 2008. PMID: 18192501 Free PMC article. - Myeloid-epithelial cross talk coordinates synthesis of the tissue-protective cytokine leukemia inhibitory factor during pneumonia.
Traber KE, Symer EM, Allen E, Kim Y, Hilliard KL, Wasserman GA, Stewart CL, Jones MR, Mizgerd JP, Quinton LJ. Traber KE, et al. Am J Physiol Lung Cell Mol Physiol. 2017 Sep 1;313(3):L548-L558. doi: 10.1152/ajplung.00482.2016. Epub 2017 May 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28522567 Free PMC article. - The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection during Pneumonia.
Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. Hilliard KL, et al. Am J Respir Cell Mol Biol. 2015 Sep;53(3):378-90. doi: 10.1165/rcmb.2014-0195OC. Am J Respir Cell Mol Biol. 2015. PMID: 25607543 Free PMC article. - Activation of Hepatic STAT3 Maintains Pulmonary Defense during Endotoxemia.
Hilliard KL, Allen E, Traber KE, Kim Y, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. Hilliard KL, et al. Infect Immun. 2015 Oct;83(10):4015-27. doi: 10.1128/IAI.00464-15. Epub 2015 Jul 27. Infect Immun. 2015. PMID: 26216424 Free PMC article. - LIF signaling in stem cells and development.
Onishi K, Zandstra PW. Onishi K, et al. Development. 2015 Jul 1;142(13):2230-6. doi: 10.1242/dev.117598. Development. 2015. PMID: 26130754 Review.
Cited by
- Multi-cohort study on cytokine and chemokine profiles in the progression of COVID-19.
Huang C, Hu X, Wang D, Gong R, Wang Q, Ren F, Wu Y, Chen J, Xiong X, Li H, Wang Q, Long G, Zhang D, Han Y. Huang C, et al. Sci Rep. 2024 May 6;14(1):10324. doi: 10.1038/s41598-024-61133-z. Sci Rep. 2024. PMID: 38710800 Free PMC article. - Respiratory infection and the impact of pulmonary immunity on lung health and disease.
Mizgerd JP. Mizgerd JP. Am J Respir Crit Care Med. 2012 Nov 1;186(9):824-9. doi: 10.1164/rccm.201206-1063PP. Epub 2012 Jul 12. Am J Respir Crit Care Med. 2012. PMID: 22798317 Free PMC article. - Roles of interleukin-11 during acute bacterial pneumonia.
Traber KE, Dimbo EL, Symer EM, Korkmaz FT, Jones MR, Mizgerd JP, Quinton LJ. Traber KE, et al. PLoS One. 2019 Aug 15;14(8):e0221029. doi: 10.1371/journal.pone.0221029. eCollection 2019. PLoS One. 2019. PMID: 31415618 Free PMC article. - CoSpar identifies early cell fate biases from single-cell transcriptomic and lineage information.
Wang SW, Herriges MJ, Hurley K, Kotton DN, Klein AM. Wang SW, et al. Nat Biotechnol. 2022 Jul;40(7):1066-1074. doi: 10.1038/s41587-022-01209-1. Epub 2022 Feb 21. Nat Biotechnol. 2022. PMID: 35190690 - MEDI3902 Correlates of Protection against Severe Pseudomonas aeruginosa Pneumonia in a Rabbit Acute Pneumonia Model.
Le HN, Quetz JS, Tran VG, Le VTM, Aguiar-Alves F, Pinheiro MG, Cheng L, Yu L, Sellman BR, Stover CK, DiGiandomenico A, Diep BA. Le HN, et al. Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02565-17. doi: 10.1128/AAC.02565-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29483116 Free PMC article.
References
- Michaud CM, Murray CJ, Bloom BR. Burden of disease--implications for future research. JAMA. 2001;285:535–539. - PubMed
- Quinton LJ, Mizgerd JP. NF-kappaB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL104053/HL/NHLBI NIH HHS/United States
- R01-HL079392/HL/NHLBI NIH HHS/United States
- R00 HL092956-04/HL/NHLBI NIH HHS/United States
- R00 HL092956/HL/NHLBI NIH HHS/United States
- R01 HL111449/HL/NHLBI NIH HHS/United States
- R00-HL092956/HL/NHLBI NIH HHS/United States
- R01 HL079392/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous