nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer - PubMed (original) (raw)
nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer
Kristopher K Frese et al. Cancer Discov. 2012 Mar.
Abstract
Nanoparticle albumin-bound (nab)-paclitaxel, an albumin-stabilized paclitaxel formulation, demonstrates clinical activity when administered in combination with gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma (PDA). The limited availability of patient tissue and exquisite sensitivity of xenografts to chemotherapeutics have limited our ability to address the mechanistic basis of this treatment regimen. Here, we used a mouse model of PDA to show that the coadministration of nab-paclitaxel and gemcitabine uniquely demonstrates evidence of tumor regression. Combination treatment increases intratumoral gemcitabine levels attributable to a marked decrease in the primary gemcitabine metabolizing enzyme, cytidine deaminase. Correspondingly, paclitaxel reduced the levels of cytidine deaminase protein in cultured cells through reactive oxygen species-mediated degradation, resulting in the increased stabilization of gemcitabine. Our findings support the concept that suboptimal intratumoral concentrations of gemcitabine represent a crucial mechanism of therapeutic resistance in PDA and highlight the advantages of genetically engineered mouse models in preclinical therapeutic trials.
Significance: This study provides mechanistic insight into the clinical cooperation observed between gemcitabine and nab-paclitaxel in the treatment of pancreatic cancer.
©2012 AACR
Figures
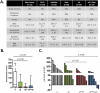
Figure 1. _nab_-paclitaxel slows tumor growth, improves survival, and decreases metastasis
A) The percentage of mice that survived for 8 days, exhibited at least one metastasis, or developed ascites was quantified. Analysis of terminal blood draws were used to measure white blood cell count, neutrophil/granulocyte count, platelet count, and hemoblogin. Normal ranges for healthy littermate non-tumor bearing mice as well as untreated KPC mice are listed. NA = not applicable, ND = not determined. B) Liver metastasis score was quantified by factoring the number and size of metastases throughout the liver. Please see materials and methods for additional information. (n≥9) C) Waterfall plot of tumor response of individual tumors from each cohort. _nab_-paclitaxel monotherapy is significantly better than vehicle, but not gemcitabine (p=0.006 and p=0.120, respectively).
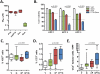
Figure 2. _nab_-paclitaxel targets the tumor epithelial cells
A) 8 KPC cell lines were exposed to a dose range of paclitaxel, _nab_-paclitaxel, docetaxel, or gemcitabine for 3 days to determine the GI50 of each agent. Data is representative of four independent experiments. B) 3 KPC cell lines were exposed to sub-GI50 levels of agents. Cells were pre-treated with DMSO or 10μM paclitaxel for 24 hours and/or treated with 30nM gemcitabine for 2 days. Data is representative of two independent experiments. The dotted lines represent predicted additive effect of combination therapy. Proliferation (C) and apoptosis (D) in tumors was measured via quantitative immunohistochemistry for Ki67 and cleaved caspase 3, respectively. (n=8) E) 10-20 high powered fields per tumor were quantified by performing co-immunofluorescence for cleaved caspase 3 and E-cadherin. (n≥9)
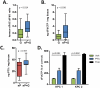
Figure 3. _nab_-paclitaxel promotes elevated intratumoral gemcitabine levels
A) The dFdC:dFdU ratio in bulk tumor was quantified in mice 2 hours after the last dose of gemcitabine. (n≥12) B) Intratumoral levels of dFdCTP were measured in duplicate samples from mice in each cohort 2 hours after the last dose of gemcitabine. (n≥12) C) Intratumoral levels of paclitaxel were measured in samples from mice in each cohort 4 hours after the last dose of _nab_-paclitaxel. (n≥7) D) 2 KPC cell lines were pre-treated with 10μM paclitaxel or DMSO for 36 hours or 10μM THU (cytidine deaminase inhibitor) for 30 minutes as a positive control and incubated with 1μM gemcitabine for 2 hours. dFdCTP levels were then measured. Data is representative of three independent experiments.
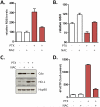
Figure 4. _nab_-paclitaxel and paclitaxel destabilizes cytidine deaminase protein
A) 40μg of bulk tumor cell lysates were immunoblotted for indicated proteins. B) Immunohistochemistry for cytidine deaminase reveals reduced protein levels in tumour epithelial cells. Scale bar = 50 μm. (n=8) C) RNA isolated from 5 KPC cell lines treated for 36 hours with 10μM paclitaxel was subjected to qRT-PCR, revealing no alterations in mRNA levels compared to controls. RQ values were generated using actin as an endogenous control. D) Protein lysates were generated from the same 5 KPC cell lines treated for 36 hours with DMSO or 10μM paclitaxel and immunoblotted for indicated proteins. Data is representative of four independent experiments. E) Protein lysates were generated from KPC cells pre-treated for 36 hours with DMSO or 10μM paclitaxel followed by 10μM MG132 for 0, 3, 10, or 30 minutes.
Figure 5
Paclitaxel inactivates cytidine deaminase through induction of reactive oxygen species (ROS). KPC cells were pretreated with 10μM paclitaxel and/or 5mM N-acetylcysteine (NAC) for 4 hours and A) incubated with CM-H2DCFDA to assess intracellular ROS via flow cytometry (n=3) or B) assessed for intracellular redox state via GSH-Glo. (n=3) C) Protein lysates were generated from KPC cells treated for 36 hours with 10μM paclitaxel and/or 5mM NAC and immunoblotted for indicated proteins. D) KPC cells were pretreated with 10μM paclitaxel and/or 5mM NAC for 36 hours and incubated with 1μM gemcitabine for 1 hour. Intracellular dFdCTP was measured (n=3).
Comment in
- Drug interactions: the importance of looking inside cancer cells.
Clark JW. Clark JW. Cancer Discov. 2012 Mar;2(3):208-10. doi: 10.1158/2159-8290.CD-12-0040. Cancer Discov. 2012. PMID: 22585991 - [nab-Paclitaxel as new therapeutic option for pancreatic cancer].
Michl P. Michl P. Z Gastroenterol. 2013 Nov;51(11):1329-30. doi: 10.1055/s-0033-1350545. Epub 2013 Nov 15. Z Gastroenterol. 2013. PMID: 24243574 German. No abstract available.
Similar articles
- Drug interactions: the importance of looking inside cancer cells.
Clark JW. Clark JW. Cancer Discov. 2012 Mar;2(3):208-10. doi: 10.1158/2159-8290.CD-12-0040. Cancer Discov. 2012. PMID: 22585991 - [nab-Paclitaxel as new therapeutic option for pancreatic cancer].
Michl P. Michl P. Z Gastroenterol. 2013 Nov;51(11):1329-30. doi: 10.1055/s-0033-1350545. Epub 2013 Nov 15. Z Gastroenterol. 2013. PMID: 24243574 German. No abstract available. - Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer.
Awasthi N, Zhang C, Schwarz AM, Hinz S, Wang C, Williams NS, Schwarz MA, Schwarz RE. Awasthi N, et al. Carcinogenesis. 2013 Oct;34(10):2361-9. doi: 10.1093/carcin/bgt227. Epub 2013 Jun 26. Carcinogenesis. 2013. PMID: 23803690 Free PMC article. - Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies.
Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Heinemann V, et al. Cancer Treat Rev. 2014 Feb;40(1):118-28. doi: 10.1016/j.ctrv.2013.04.004. Epub 2013 Jul 9. Cancer Treat Rev. 2014. PMID: 23849556 Review. - Treatment of metastatic pancreatic adenocarcinoma: a review.
Thota R, Pauff JM, Berlin JD. Thota R, et al. Oncology (Williston Park). 2014 Jan;28(1):70-4. Oncology (Williston Park). 2014. PMID: 24683721 Review.
Cited by
- CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer.
Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. Neesse A, et al. Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12325-30. doi: 10.1073/pnas.1300415110. Epub 2013 Jul 8. Proc Natl Acad Sci U S A. 2013. PMID: 23836645 Free PMC article. - Tailor-Made Nanomaterials for Diagnosis and Therapy of Pancreatic Ductal Adenocarcinoma.
Hu X, Xia F, Lee J, Li F, Lu X, Zhuo X, Nie G, Ling D. Hu X, et al. Adv Sci (Weinh). 2021 Feb 12;8(7):2002545. doi: 10.1002/advs.202002545. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33854877 Free PMC article. Review. - Stromal disrupting effects of nab-paclitaxel in pancreatic cancer.
Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M. Alvarez R, et al. Br J Cancer. 2013 Aug 20;109(4):926-33. doi: 10.1038/bjc.2013.415. Epub 2013 Aug 1. Br J Cancer. 2013. PMID: 23907428 Free PMC article. - Targeting inflammation in pancreatic cancer: Clinical translation.
Steele CW, Kaur Gill NA, Jamieson NB, Carter CR. Steele CW, et al. World J Gastrointest Oncol. 2016 Apr 15;8(4):380-8. doi: 10.4251/wjgo.v8.i4.380. World J Gastrointest Oncol. 2016. PMID: 27096033 Free PMC article. Review. - Intracellular Cytidine Deaminase Regulates Gemcitabine Metabolism in Pancreatic Cancer Cell Lines.
Bjånes TK, Jordheim LP, Schjøtt J, Kamceva T, Cros-Perrial E, Langer A, Ruiz de Garibay G, Kotopoulis S, McCormack E, Riedel B. Bjånes TK, et al. Drug Metab Dispos. 2020 Mar;48(3):153-158. doi: 10.1124/dmd.119.089334. Epub 2019 Dec 23. Drug Metab Dispos. 2020. PMID: 31871136 Free PMC article.
References
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. - PubMed
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. - PubMed
- Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases