Identification of the rostral migratory stream in the canine and feline brain - PubMed (original) (raw)
Identification of the rostral migratory stream in the canine and feline brain
Saafan Z Malik et al. PLoS One. 2012.
Abstract
In the adult rodent brain, neural progenitor cells migrate from the subventricular zone of the lateral ventricle towards the olfactory bulb in a track known as the rostral migratory stream (RMS). To facilitate the study of neural progenitor cells and stem cell therapy in large animal models of CNS disease, we now report the location and characteristics of the normal canine and feline RMS. The RMS was found in Nissl-stained sagittal sections of adult canine and feline brains as a prominent, dense, continuous cellular track beginning at the base of the anterior horn of the lateral ventricle, curving around the head of the caudate nucleus and continuing laterally and ventrally to the olfactory peduncle before entering the olfactory tract and bulb. To determine if cells in the RMS were proliferating, the thymidine analog 5-bromo-2-deoxyuridine (BrdU) was administered and detected by immunostaining. BrdU-immunoreactive cells were present throughout this track. The RMS was also immunoreactive for markers of proliferating cells, progenitor cells and immature neurons (Ki-67 and doublecortin), but not for NeuN, a marker of mature neurons. Luxol fast blue and CNPase staining indicated that myelin is closely apposed to the RMS along much of its length and may provide guidance cues for the migrating cells. Identification and characterization of the RMS in canine and feline brain will facilitate studies of neural progenitor cell biology and migration in large animal models of neurologic disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Rostral migratory stream in the dog brain.
(A) Schematic of a sagittal view of a canine brain. The red line indicates the location of the RMS in relationship to the anterior horm of the lateral ventricle (LV), caudate nucleus (CN), olfactory bulb (OB), cortex (Ctx) and cerebellum (Cb). (B) Nissl staining showing the orientation and nomenclature of the canine RMS. (C) anti-BrdU immunostaining in brown with hematoxylin counterstain in purple. C1 shows BrdU staining at the boundary of the white matter and caudate nucleus in the descending limb; C2 shows BrdU staining in the olfactory peduncle/rostral limb. (D) anti-Dcx immunostaining. D1 shows morphology of cells in the funnel; D2 shows leading and trailing processes of migrating cells in the descending limb. (E) Immunoreactivity for BrdU (green, in the RMS) and NeuN (red, in the CN) does not overlap in the descending limb. Section is counterstained with DAPI (blue). (F) BrdU immunostaining (brown) in the olfactory peduncle in tissue from dog 5, analyzed at 6 hr after a single 75 mg/kg i.v., indicating that BrdU is taken up by dividing cells all along the RMS. Scale bar in B, C, D: 1 mm. Scale bars in C1, C2, E: 100 microns. Scale bars in D1, D2, F: 50 microns. Please view the figures on a computer monitor for accurate RGB color representation.
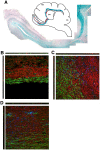
Figure 2. Rostral migratory stream in the cat brain.
(A) Nissl staining demonstrating the RMS orientation and location from the anterior horn of the lateral ventricle (LV) to the olfactory bulb (OB). (B) anti-BrdU immunostaining. (C) anti-Ki67 immunostaining, demonstrating the presence of dividing cells along the entire RMS. (D, E) T2-weighted MRI images of a cat head in the dorsal (D) and transverse (E) planes, showing cerebrospinal fluid in the open olfactory ventricles of an adult (5 year old) cat (arrows). Scale bars in main panels A–D: 500 microns. Scale bars in insets B1–2, C1–3∶100 microns.
Figure 3. Relationship of white matter and the RMS.
(A) Luxol fast blue staining of a saggital section from dog 4. Inset shows the approximate location of the RMS in red and part of the white matter in blue. (B–D) Confocal maximum projection images of CNPase staining (red) and doublecortin staining (green) in the SVZ (B), funnel (C), and olfactory peduncle (D) in dog 1.
Figure 4. Areas of dog brain (A–C) and cat brain (D–E) embedded for sectioning.
(A, D): Lateral view. (C, E): Ventral view. (B): View from the midline. Hemispheres were separated and an ∼40×22×22 mm block (including the olfactory bulb when possible) was isolated from each side (B’–E’). Scale bar in B’ and D’ is 1 cm. Labels are based on the atlas of Singer.
Similar articles
- Morphological and Cellular Characterization of the Fetal Canine (Canis lupus familiaris) Subventricular Zone, Rostral Migratory Stream, and Olfactory Bulb.
Orechio D, Andrade Aguiar B, Baroni Diniz G, Cioni Bittencourt J, Haemmerle CAS, Watanabe IS, Miglino MA, Castelucci P. Orechio D, et al. Anat Rec (Hoboken). 2018 Sep;301(9):1570-1584. doi: 10.1002/ar.23855. Epub 2018 Jul 2. Anat Rec (Hoboken). 2018. PMID: 29752870 - The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2.
Bédard A, Lévesque M, Bernier PJ, Parent A. Bédard A, et al. Eur J Neurosci. 2002 Nov;16(10):1917-24. doi: 10.1046/j.1460-9568.2002.02263.x. Eur J Neurosci. 2002. PMID: 12453055 - Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain.
Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Pencea V, et al. Exp Neurol. 2001 Nov;172(1):1-16. doi: 10.1006/exnr.2001.7768. Exp Neurol. 2001. PMID: 11681836 - Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.
Khodosevich K, Alfonso J, Monyer H. Khodosevich K, et al. Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review. - Regulation of subventricular zone-derived cells migration in the adult brain.
Capilla-Gonzalez V, Lavell E, Quiñones-Hinojosa A, Guerrero-Cazares H. Capilla-Gonzalez V, et al. Adv Exp Med Biol. 2015;853:1-21. doi: 10.1007/978-3-319-16537-0_1. Adv Exp Med Biol. 2015. PMID: 25895704 Review.
Cited by
- The Olfactory Bulb in Companion Animals-Anatomy, Physiology, and Clinical Importance.
Alvites R, Caine A, Cherubini GB, Prada J, Varejão ASP, Maurício AC. Alvites R, et al. Brain Sci. 2023 Apr 24;13(5):713. doi: 10.3390/brainsci13050713. Brain Sci. 2023. PMID: 37239185 Free PMC article. Review. - Bioengineering the neurovascular niche to study the interaction of neural stem cells and endothelial cells.
Winkelman MA, Koppes AN, Koppes RA, Dai G. Winkelman MA, et al. APL Bioeng. 2021 Mar 3;5(1):011507. doi: 10.1063/5.0027211. eCollection 2021 Mar. APL Bioeng. 2021. PMID: 33688617 Free PMC article. Review. - Diving into the streams and waves of constitutive and regenerative olfactory neurogenesis: insights from zebrafish.
Calvo-Ochoa E, Byrd-Jacobs CA, Fuss SH. Calvo-Ochoa E, et al. Cell Tissue Res. 2021 Jan;383(1):227-253. doi: 10.1007/s00441-020-03334-2. Epub 2020 Nov 27. Cell Tissue Res. 2021. PMID: 33245413 Review. - Revisit the Candidacy of Brain Cell Types as the Cell(s) of Origin for Human High-Grade Glioma.
Shao F, Liu C. Shao F, et al. Front Mol Neurosci. 2018 Feb 21;11:48. doi: 10.3389/fnmol.2018.00048. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29515370 Free PMC article. - Human adult neurogenesis across the ages: An immunohistochemical study.
Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Dennis CV, et al. Neuropathol Appl Neurobiol. 2016 Dec;42(7):621-638. doi: 10.1111/nan.12337. Epub 2016 Aug 28. Neuropathol Appl Neurobiol. 2016. PMID: 27424496 Free PMC article.
References
- Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nature Reviews Neuroscience. 2006;7:75–84. - PubMed
- Lipsitz D, Higgins RJ, Kortz GD, Dickinson PJ, Bollen AW, et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40:659–669. - PubMed
- Stoica G, Kim HT, Hall DG, Coates JR. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41:10–19. - PubMed
- Thomson SA, Kennerly E, Olby N, Mickelson JR, Hoffmann DE, et al. Microarray analysis of differentially expressed genes of primary tumors in the canine central nervous system. Vet Pathol. 2005;42:550–558. - PubMed
Publication types
MeSH terms
Grants and funding
- DK25759/DK/NIDDK NIH HHS/United States
- RR02512/RR/NCRR NIH HHS/United States
- P40 RR002512/RR/NCRR NIH HHS/United States
- R01 DK054481/DK/NIDDK NIH HHS/United States
- R01 DK025759/DK/NIDDK NIH HHS/United States
- DK54481/DK/NIDDK NIH HHS/United States
- P40 OD010939/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources