Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats - PubMed (original) (raw)
Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats
Yuen-Shan Ho et al. PLoS One. 2012.
Abstract
Cigarette smoking has been proposed as a major risk factor for aging-related pathological changes and Alzheimer's disease (AD). To date, little is known for how smoking can predispose our brains to dementia or cognitive impairment. This study aimed to investigate the cigarette smoke-induced pathological changes in brains. Male Sprague-Dawley (SD) rats were exposed to either sham air or 4% cigarette smoke 1 hour per day for 8 weeks in a ventilated smoking chamber to mimic the situation of chronic passive smoking. We found that the levels of oxidative stress were significantly increased in the hippocampus of the smoking group. Smoking also affected the synapse through reducing the expression of pre-synaptic proteins including synaptophysin and synapsin-1, while there were no changes in the expression of postsynaptic protein PSD95. Decreased levels of acetylated-tubulin and increased levels of phosphorylated-tau at 231, 205 and 404 epitopes were also observed in the hippocampus of the smoking rats. These results suggested that axonal transport machinery might be impaired, and the stability of cytoskeleton might be affected by smoking. Moreover, smoking affected amyloid precursor protein (APP) processing by increasing the production of sAPPβ and accumulation of β-amyloid peptide in the CA3 and dentate gyrus region. In summary, our data suggested that chronic cigarette smoking could induce synaptic changes and other neuropathological alterations. These changes might serve as evidence of early phases of neurodegeneration and may explain why smoking can predispose brains to AD and dementia.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
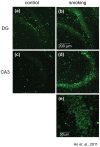
Figure 1. Level of 8-OHG was elevated in rat hippocampus exposed to cigarette smoking.
Brain sections were stained with anti-8-OHG antibody to detect the level of oxidative stress. Representative photos were selected from the control group (a and c) and the smoking group (b and d), magnification, 100X. An enlarged photo of the CA3 region of the smoking group was shown in (e), magnification, 400X.
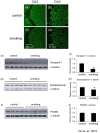
Figure 2. Levels of the pre-synaptic proteins were decreased in the hippocampus of the smoking group.
Brain sections were stained with anti-synapsin-1 antibody. The CA3 region of (a) control group and (c) smoking group were shown, magnification, 200X; with corresponding enlarged images shown in (b and d), magnification, 400X. Western blot analysis was performed on the total lysate of the whole hippocampus, confirming the decrease of synapsin-1 in the hippocampus of the smoking group (e); quantitative analysis of the band intensity of synapsin-1 was shown in (f). Western blot analysis showed that the levels of synaptophysin in the hippocampus were decreased in the smoking group (f); quantitative analysis of the band intensity of synaptophysin was shown in (g). Western blot analysis showed that the levels of PSD95 in the hippocampus were similar among the two groups (h); quantitative analysis of the band intensity of PSD95 was shown in (i). *P<0.05 compared to control.
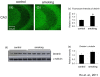
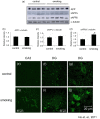
Figure 3. Level of drebrin was increased in the smoking group. Brain sections were stained with anti-drebrin antibody.
The CA3 region of the control (a) and smoking (b) group were shown, magnification, 100X. Western blot analysis showed that there was a trend of increase in the band intensity of drebrin but it is not statistically significant (c and d).
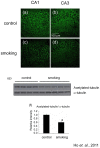
Figure 4. Level of acetylated-tubulin was decreased in the hippocampus of the smoking group.
Brain sections were stained with anti-acetylated-tubulin antibody. The CA1 and CA3 regions of control group (a and b), smoking group (c and d) were shown, magnification, 200X. Western blot analysis confirmed the decrease of acetylated-tubulin in the hippocampus of the smoking group (e); quantitative analysis of the band intensity was shown in (f). *P<0.05 compared to control.
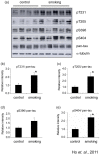
Figure 5. Smoking induced phosphorylation of tau.
The total lysate of the hippocampus of the rats were subjected to Western blotting analysis. Phosphorylation of tau was detected by pT231 (reacts with phosphorylated tau at Thr 231), pT205 (reacts with phosphorylated tau at Thr 205), pS396 (reacts with phosphorylated tau at Ser 396) and pS404 (reacts with phosphorylated tau at Ser 404). Total tau was detected with the antibody pan-tau. α-tubulin was used as loading control. *P<0.05 compared to control.
Figure 6. Smoking induced alteration of APP processing.
The total lysate of the hippocampus of the rats were subjected to Western blotting analysis. The levels of APP, sAPPα and sAPPβ were detected (a); quantitative analysis of the band intensity was shown (b to d). *P<0.05 compared to control. Brain sections were stained with anti-rodent Aβ antibody. Photos of the CA1 and dentate gyrus were presented. Positive staining were increased in the smoking group (h and i) when compared to the control group (e and f), magnification, 400X. Enlarged photos of the dentate gyrus of the control (g) and smoking group (j) were shown. Note that Aβ was accumulated in the cell body (j).
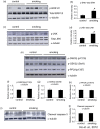
Figure 7. Smoking induced activation of stress kinases.
The total lysate of the hippocampus of the rats were subjected to Western blotting analysis. The levels of p-ERK1/2, total ERK1/2 (a), p-JNK and total JNK (b), p-GSK3β (Ser9), p-GSK3β (pY216), p-PP2A (pY307) (e) and cleaved caspase-3 (i) were detected. α-Tubulin was used as loading control. Quantitative analysis of the band intensity of the detected kinases was shown in (b, d, f–g, and j). *P<0.05 compared to control.
Similar articles
- Alzheimer's disease/dementia-associated brain pathology in aging DPP6-KO mice.
Lin L, Petralia RS, Holtzclaw L, Wang YX, Abebe D, Hoffman DA. Lin L, et al. Neurobiol Dis. 2022 Nov;174:105887. doi: 10.1016/j.nbd.2022.105887. Epub 2022 Oct 6. Neurobiol Dis. 2022. PMID: 36209950 Free PMC article. - Region-specific expression of tau, amyloid-β protein precursor, and synaptic proteins at physiological condition or under endoplasmic reticulum stress in rats.
Lin L, Yang SS, Chu J, Wang L, Ning LN, Zhang T, Jiang Q, Tian Q, Wang JZ. Lin L, et al. J Alzheimers Dis. 2014;41(4):1149-63. doi: 10.3233/JAD-140207. J Alzheimers Dis. 2014. PMID: 24787918 - Normalizing the gene dosage of Dyrk1A in a mouse model of Down syndrome rescues several Alzheimer's disease phenotypes.
García-Cerro S, Rueda N, Vidal V, Lantigua S, Martínez-Cué C. García-Cerro S, et al. Neurobiol Dis. 2017 Oct;106:76-88. doi: 10.1016/j.nbd.2017.06.010. Epub 2017 Jun 21. Neurobiol Dis. 2017. PMID: 28647555 - Alzheimer's disease and amyloid: culprit or coincidence?
Skaper SD. Skaper SD. Int Rev Neurobiol. 2012;102:277-316. doi: 10.1016/B978-0-12-386986-9.00011-9. Int Rev Neurobiol. 2012. PMID: 22748834 Review. - Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.
Terwel D, Dewachter I, Van Leuven F. Terwel D, et al. Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review.
Cited by
- Cohort study of the effects of occupation and environmental tobacco smoke on the incidence of Alzheimer's disease among seniors.
Yang L, Wan W, Xuan C, Yu C, Jin K, Zheng P, Yan J. Yang L, et al. Tob Induc Dis. 2023 Feb 6;21:18. doi: 10.18332/tid/157208. eCollection 2023. Tob Induc Dis. 2023. PMID: 36762262 Free PMC article. Review. - Changes in metabolic hormones and trace elements in CSF in active smokers indicate oxidative damage to brain cells.
Zheng P, Wang F, Li H, Chen H, Li M, Ma H, He J, Chen L, Liu Y, Xu H. Zheng P, et al. Endocr Connect. 2024 May 27;13(6):e240016. doi: 10.1530/EC-24-0016. Print 2024 Jun 1. Endocr Connect. 2024. PMID: 38688314 Free PMC article. - Neuroinflammation, Oxidative Stress, Apoptosis, Microgliosis and Astrogliosis in the Cerebellum of Mice Chronically Exposed to Waterpipe Smoke.
Hamadi N, Beegam S, Zaaba NE, Elzaki O, Altamimi MA, Nemmar A. Hamadi N, et al. Biomedicines. 2023 Apr 6;11(4):1104. doi: 10.3390/biomedicines11041104. Biomedicines. 2023. PMID: 37189722 Free PMC article. - Nicotine inhibits the proliferation by upregulation of nitric oxide and increased HDAC1 in mouse neural stem cells.
Lee H, Park JR, Yang J, Kim E, Hong SH, Woo HM, Ryu SM, Cho SJ, Park SM, Yang SR. Lee H, et al. In Vitro Cell Dev Biol Anim. 2014 Sep;50(8):731-9. doi: 10.1007/s11626-014-9763-0. Epub 2014 May 2. In Vitro Cell Dev Biol Anim. 2014. PMID: 24789730 - Links between the Brain and Retina: The Effects of Cigarette Smoking-Induced Age-Related Changes in Alzheimer's Disease and Macular Degeneration.
Yu SS, Tang X, Ho YS, Chang RC, Chiu K. Yu SS, et al. Front Neurol. 2016 Jul 27;7:119. doi: 10.3389/fneur.2016.00119. eCollection 2016. Front Neurol. 2016. PMID: 27512384 Free PMC article. No abstract available.
References
- Anstey KJ, von SC, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. - PubMed
- Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004;62:920–924. - PubMed
- Ott A, Slooter AJ, Hofman A, van HF, Witteman JC, et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous