Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages - PubMed (original) (raw)
Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages
Paolo S Manzanillo et al. Cell Host Microbe. 2012.
Abstract
Cytosolic bacterial pathogens activate the cytosolic surveillance pathway (CSP) and induce innate immune responses, but how the host detects vacuolar pathogens like Mycobacterium tuberculosis is poorly understood. We show that M. tuberculosis also initiates the CSP upon macrophage infection via limited perforation of the phagosome membrane mediated by the ESX-1 secretion system. Although the bacterium remains within the phagosome, this permeabilization results in phagosomal and cytoplasmic mixing and allows extracellular mycobacterial DNA to access host cytosolic receptors, thus blurring the distinction between "vacuolar" and "cytosolic" pathogens. Activation of cytosolic receptors induces signaling through the Sting/Tbk1/Irf3 axis, resulting in IFN-β production. Surprisingly, Irf3(-/-) mice, which cannot respond to cytosolic DNA, are resistant to long-term M. tuberculosis infection, suggesting that the CSP promotes M. tuberculosis infection. Thus, cytosolic sensing of mycobacterial DNA plays a key role in M. tuberculosis pathogenesis and likely contributes to the high type I IFN signature in tuberculosis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
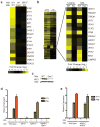
Figure 1. M. tuberculosis elicits the CSP via ESX-1 secretion
(A) Microarray analysis of CSP-regulated genes in wild-type and IRF3−/− BMDMs infected with the indicated M. tuberculosis strains. Log2 fold induction versus uninfected macrophages is shown. (B) Cluster analysis of BMDM genes induced during infection with wild-type (WT) or Δ_hly L. monocytogenes_ (_hly_−), and with wild-type or ESX-1 mutant (Esx1−) M. tuberculosis. (C) Nuclear translocation of IRF3 in BMDMs 3 h post-infection was assessed by western blotting of nuclear fractions using IRF3 and TATA-binding protein (TBP)-specific antibodies. (D and E) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR 3 h post-infection in BMDMs (MΦ) and normalized to actin. TBK1−/− mice are viable only when TNFα signaling is abrogated, thus BMDMs from TNFR1−/− mice serve as the control for this strain. Data shown is the mean ± SD, (N=3 per group). *P<0.001 as determined by Student’s t-test comparing gene expression in each mutant macrophage and its corresponding control. See Figure S1 and Table S1 for a detailed time course analysis of CSP gene induction and microarray expression data.
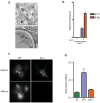
Figure 2. M. tuberculosis permeabilizes the phagosomal membrane early after infection
(A) Electron microscopy of C57/B6 BMDMs infected with wild-type M. tuberculosis 3 h post-infection. Macrophage cytosol (MΦ), M. tuberculosis cell (tb) and intact phagosomal membrane (arrows) are indicated. (B) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR (actin normalized) in BMDMs infected with Δ_esxAM. tuberculosis_ cells containing an LLO expression plasmid or empty vector. Data shown is the mean ± SD, (N=3 per group). *P<0.001 as determined by Student’s t-test comparing gene expression in each mutant macrophage and its corresponding control. (C) Macrophages were preloaded with CCF4-am substrate and infected with wild-type or Δ_esxAM. tuberculosis_ strains, both of which over-expressed the blaC gene. 3 h post infection, cells were excited with a 405nm laser, and percent CCF4 cleavage was measured as the ratio of 450nm:535nm emission by fluorescence microscopy of infected cells (D). Data shown is the mean ± SD, (N=3 per group).
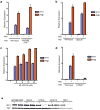
Figure 3. M. tuberculosis activates the STING/TBK1/IRF3 pathway
(A, B) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR in BMDMs (MΦ) as in Figure 1. Data shown is the mean ± SD, (N≥3 per group). (C) Wild-type C57L/B6 BMDMs were pre-treated for two-hours with the RNA polymerase III inhibitor ML-60218 and infected with wild-type M. tuberculosis for 3 hours prior to RNA extraction. Data shown is the mean ± SD, (N=3 per group). (D) IFIT1 and IFN-β mRNA levels were assessed 3 h post-infection of STING−/− macrophages wild-type M. tuberculosis. Data shown is the mean ± SD, (N=3 per group). *P<0.001 as determined by Student’s t-test. (E) Nuclear translocation of IRF3 in BMDMs was determined as described in Figure 1C.
Figure 4. The cytosolic DNA sensor IFI204/IFI16 contributes to CSP activation
(A) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR in BMDMs (MΦ) from wild-type and DAI knockout mice infected with wild-type M. tuberculosis for 3 h. Data shown is the mean ± SD, (N=3 per group). (B) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR after M. tuberculosis infection of immortalized BMDMs (iBMDMs) transduced with lentiviral constructs expressing one of two different shRNAs targeting IFI204 (IFI204_–_1 and IFI204_–_2) or a scrambled shRNA control. Data shown is the mean ± SD, (N=3 per group). *P<0.005 by Student’s T-test. (C) IFI204 mRNA levels were assessed by qRT-PCR in the transduced iBMDMs described in (B). *P<0.005 by Student’s T-test. (D) Wild-type C57L/B6 BMDMswere infected with either wild-type or Δ_esxAM. tuberculosis_ for 3 hours and cellular localization of IFI204 as assessed via immunofluorescence. See Table S2 for shRNA sequences and primers used for this study.
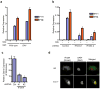
Figure 5. Extracellular M. tuberculosis DNA triggers the CSP
(A) IFIT1 and IFN-β mRNA levels were assessed by qRT-PCR in infected BMDMs (MΦ) as described in Figure 1. (B) RAW264.7 cells were stably transfected with a plasmid over-expressing either Trex1 or LacZ and infected with wild-type M. tuberculosis for 4 h. IFIT1 and IFN-β gene expression was analyzed by qRT-PCR. (C) Luminescence readings from BMDMs infected with either wild-type (Wt), ESX-1 mutant (Esx1−), or complemented ESX-1 mutant (Comp) M. tuberculosis carrying either empty vector or a plasmid encoding a secreted form of Metridia luciferase under the control of the eukaryotic-specific CMV promoter (pMAN4). Luminescence readings were taken at 16 h post infection. (D) M. tuberculosis cells were labeled with BrdU prior to macrophage infection. After 3 h, macrophage cytosolic fractions were isolated and BrdU-labeled bacterial DNA was recovered by immunoprecipitation using anti-BrdU antibodies, and separated by agarose gel electrophoresis. (E) Lysates from (D) were used as templates for qPCR quantification of M. tuberculosis DNA. Data shown for (A) (B) and (D) is the mean ± SD, (N=3 per group). *P<0.01 by Student’s T-test, comparing mutant and overexpression strains to their corresponding controls. Also see Figure S2 for analysis of M. tuberculosis c-di-nucleotide mutants in CSP activation and Table S3 for M. tuberculosis strains used in this study.
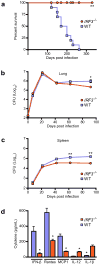
Figure 6. Cytosolic signaling is required for virulence of M. tuberculosis
(A) Kaplan-Meier survival analysis of IRF3−/− and C57BL/6 control mice infected with 102 CFU wild-type M. tuberculosis via the aerosol route (n = 10 per group). IRF3−/− mice showed significantly improved survival compared to wild-type (WT) mice as calculated by log-rank test (**P < 0.001). (B, C) Bacterial burdens as measured by colony forming units (CFU) in the lungs (B) and spleen (C) of IRF3−/− and wild-type mice infected as described in (A) (n = 5 per time point) at the indicated time points. Data shown are the mean ± SD. *P < 0.02 and **P <0.005 comparing C57BL/6 and IRF3−/− mice by Kruskal Wallis. (D) IFN-β, Rantes, MCP1, IL-12 and IL-1β levels were measured by ELISA in serum from IRF3−/− and C57BL/6 control mice 21 days post infection (n = 4 per group). Data shown is the mean ± SD. *P < 0.005 comparing C57BL/6 and IRF3−/− mice as determined by Student’s T-test.
Comment in
- Bacterial pathogenesis: TB blurs the lines.
Molloy S. Molloy S. Nat Rev Microbiol. 2012 Jun 15;10(7):442. doi: 10.1038/nrmicro2825. Nat Rev Microbiol. 2012. PMID: 22699958 No abstract available.
Similar articles
- Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway.
Watson RO, Manzanillo PS, Cox JS. Watson RO, et al. Cell. 2012 Aug 17;150(4):803-15. doi: 10.1016/j.cell.2012.06.040. Cell. 2012. PMID: 22901810 Free PMC article. - _Mycobacterium tuberculosis_-induced IFN-β production requires cytosolic DNA and RNA sensing pathways.
Cheng Y, Schorey JS. Cheng Y, et al. J Exp Med. 2018 Nov 5;215(11):2919-2935. doi: 10.1084/jem.20180508. Epub 2018 Oct 18. J Exp Med. 2018. PMID: 30337468 Free PMC article. - Cytosolic serpins act in a cytoprotective feedback loop that limits ESX-1-dependent death of _Mycobacterium marinum_-infected macrophages.
Nobs E, Laschanzky K, Munke K, Movert E, Valfridsson C, Carlsson F. Nobs E, et al. mBio. 2024 Sep 11;15(9):e0038424. doi: 10.1128/mbio.00384-24. Epub 2024 Aug 1. mBio. 2024. PMID: 39087767 Free PMC article. - Mycobacterial manipulation of vacuolar sorting.
Philips JA. Philips JA. Cell Microbiol. 2008 Dec;10(12):2408-15. doi: 10.1111/j.1462-5822.2008.01239.x. Epub 2008 Sep 8. Cell Microbiol. 2008. PMID: 18783482 Review. - Mycobacterial manipulation of the host cell.
Hestvik AL, Hmama Z, Av-Gay Y. Hestvik AL, et al. FEMS Microbiol Rev. 2005 Nov;29(5):1041-50. doi: 10.1016/j.femsre.2005.04.013. Epub 2005 Jul 1. FEMS Microbiol Rev. 2005. PMID: 16040149 Review.
Cited by
- STING and the innate immune response to nucleic acids in the cytosol.
Burdette DL, Vance RE. Burdette DL, et al. Nat Immunol. 2013 Jan;14(1):19-26. doi: 10.1038/ni.2491. Nat Immunol. 2013. PMID: 23238760 Review. - BBQ methods: streamlined workflows for bacterial burden quantification in infected cells by confocal microscopy.
Augenstreich J, Shuster M, Fan Y, Lyu Z, Ling J, Briken V. Augenstreich J, et al. Biol Open. 2024 Jan 15;13(1):bio060189. doi: 10.1242/bio.060189. Epub 2024 Jan 22. Biol Open. 2024. PMID: 38156988 Free PMC article. - A Nonsense Mutation in Mycobacterium marinum That Is Suppressible by a Novel Mechanism.
Williams EA, Mba Medie F, Bosserman RE, Johnson BK, Reyna C, Ferrell MJ, Champion MM, Abramovitch RB, Champion PA. Williams EA, et al. Infect Immun. 2017 Jan 26;85(2):e00653-16. doi: 10.1128/IAI.00653-16. Print 2017 Feb. Infect Immun. 2017. PMID: 27789543 Free PMC article. - Noncanonical roles of ATG5 and membrane atg8ylation in retromer assembly and function.
Paddar MA, Wang F, Trosdal ES, Hendrix E, He Y, Salemi M, Mudd M, Jia J, Duque TLA, Javed R, Phinney B, Deretic V. Paddar MA, et al. bioRxiv [Preprint]. 2024 Oct 14:2024.07.10.602886. doi: 10.1101/2024.07.10.602886. bioRxiv. 2024. PMID: 39026874 Free PMC article. Updated. Preprint. - Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma.
Matty MA, Roca FJ, Cronan MR, Tobin DM. Matty MA, et al. Immunol Rev. 2015 Mar;264(1):276-87. doi: 10.1111/imr.12273. Immunol Rev. 2015. PMID: 25703566 Free PMC article. Review.
References
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI076632/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- R01 AI081727/AI/NIAID NIH HHS/United States
- T32 AI060537/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous