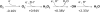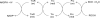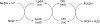Why do bacteria use so many enzymes to scavenge hydrogen peroxide? - PubMed (original) (raw)
Review
Why do bacteria use so many enzymes to scavenge hydrogen peroxide?
Surabhi Mishra et al. Arch Biochem Biophys. 2012.
Abstract
Hydrogen peroxide (H(2)O(2)) is continuously formed by the autoxidation of redox enzymes in aerobic cells, and it also enters from the environment, where it can be generated both by chemical processes and by the deliberate actions of competing organisms. Because H(2)O(2) is acutely toxic, bacteria elaborate scavenging enzymes to keep its intracellular concentration at nanomolar levels. Mutants that lack such enzymes grow poorly, suffer from high rates of mutagenesis, or even die. In order to understand how bacteria cope with oxidative stress, it is important to identify the key enzymes involved in H(2)O(2) degradation. Catalases and NADH peroxidase (Ahp) are primary scavengers in many bacteria, and their activities and physiological impacts have been unambiguously demonstrated through phenotypic analysis and through direct measurements of H(2)O(2) clearance in vivo. Yet a wide variety of additional enzymes have been proposed to serve similar roles: thiol peroxidase, bacterioferritin comigratory protein, glutathione peroxidase, cytochrome c peroxidase, and rubrerythrins. Each of these enzymes can degrade H(2)O(2) in vitro, but their contributions in vivo remain unclear. In this review we examine the genetic, genomic, regulatory, and biochemical evidence that each of these is a bonafide scavenger of H(2)O(2) in the cell. We also consider possible reasons that bacteria might require multiple enzymes to catalyze this process, including differences in substrate specificity, compartmentalization, cofactor requirements, kinetic optima, and enzyme stability. It is hoped that the resolution of these issues will lead to an understanding of stress resistance that is more accurate and perceptive.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Fig. 1
Stepwise univalent reduction of oxygen to superoxide, hydrogen peroxide, the hydroxyl radical, and water. Standard potentials are indicated for pH 7; the oxygen/superoxide potential is designated for 1 M molecular oxygen (not 1 atmosphere).
Fig. 2
A requirement for endogenous scavengers of H2O2. A. E. coli mutants that lack scavenging enzymes continuously release H2O2 into the extracellular medium. Growing cells were suspended at time zero into fresh glucose/amino-acids medium, and the concentration of extracellular H2O2 was determined at intervals using a peroxidase assay [10]. Medium was gassed either with air or with pure oxygen, as indicated. The rate of intracellular formation can be deduced to be 10–15 μM/sec in air-saturated medium. B. The same mutants are unable to grow in simple aerobic glucose medium. At time zero the strains were switched from anaerobic to aerobic media, and biomass was monitored by measurements of optical density. The mutant strain lacks both catalases and NADH peroxidase (Kat− Ahp−); it does not exhibit any defect under anaerobic conditions.
Fig. 2
A requirement for endogenous scavengers of H2O2. A. E. coli mutants that lack scavenging enzymes continuously release H2O2 into the extracellular medium. Growing cells were suspended at time zero into fresh glucose/amino-acids medium, and the concentration of extracellular H2O2 was determined at intervals using a peroxidase assay [10]. Medium was gassed either with air or with pure oxygen, as indicated. The rate of intracellular formation can be deduced to be 10–15 μM/sec in air-saturated medium. B. The same mutants are unable to grow in simple aerobic glucose medium. At time zero the strains were switched from anaerobic to aerobic media, and biomass was monitored by measurements of optical density. The mutant strain lacks both catalases and NADH peroxidase (Kat− Ahp−); it does not exhibit any defect under anaerobic conditions.
Fig. 3
Exogenous sources of H2O2 include H2O2-excreting microbes such as lactic acid bacteria, the NADPH oxidase responses of plants and macrophages, photochemically driven redox reactions, and chemical thiol/metal oxidations that occur at oxic-anoxic interfaces. Endogenous H2O2 is constantly formed by the adventitious oxidations of flavoenzymes. The H2O2 damages DNA through the Fenton reaction. It also disables dehydratases that contain iron-sulfur clusters and non-redox mononuclear enzymes that contain iron.
Fig. 4
The catalytic cycle of alkylhydroperoxide reductase (Ahp).
Fig. 5
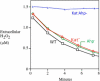
H2O2 decomposition by aerobic E. coli depends upon catalase and alkylhydroperoxide reductase (Ahp). H2O2 (1.5 μM) was added to a dilute suspension of cells at time zero, and the amount of remaining extracellular H2O2 was subsequently determined at intervals. Note that for the wild-type strain and the single mutants, the rate-limiting step in H2O2 degradation was diffusion into the cell; consequently, the rates of H2O2 clearance from the medium were equivalent. Measurements were made as described [10].
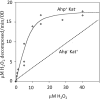
Fig. 6
Decomposition of exogenous H2O2 by E. coli Ahp+ Kat− mutants (solid line) or Ahp− Kat+ mutants (smooth line). Alkylhydroperoxide reductase (Ahp) is effective at low H2O2 concentrations, but catalase is the more effective enzyme at high concentrations. Note the difference in scale between panels A and B. Measurements were made as discussed in Fig. 5.
Fig. 6
Decomposition of exogenous H2O2 by E. coli Ahp+ Kat− mutants (solid line) or Ahp− Kat+ mutants (smooth line). Alkylhydroperoxide reductase (Ahp) is effective at low H2O2 concentrations, but catalase is the more effective enzyme at high concentrations. Note the difference in scale between panels A and B. Measurements were made as discussed in Fig. 5.
Fig. 7
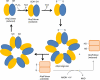
Proposed electron transfer pathway of the thiol-based peroxidases: bacterioferritin comigratory protein (BCP), thiol peroxidase (Tpx), and glutathione peroxidase (Gpx). Prx in the figure is a general representation of all three thiol based peroxidases. Trx and TrxR represents thioredoxin and thioredoxin reductase, respectively.
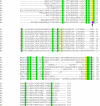
Fig. 8
Multiple sequence alignment showing conservation of glutathione peroxidase (Gpx) active-site residues (yellow) within various bacteria (Ec: E. coli; Se: Salmonella enteric serovar Typhimurium; Pa: Pseudomonas aeruginosa; Bs: Bacillus subtilis 168; Nm: Neisseria meningitides; Sy: Synechocystis sp.; Bj: Bradyrhizobium japonicum; Rs: Rhodobacter sphaeroides; Bf: Bacteroides fragilis) and human glutathione peroxidase I (Hs). The presence of selenocysteine in place of cysteine in human Gpx 1 has been marked by an arrow. Other conserved residues are highlighted in green.
Fig. 9
Proposed electron-transfer pathway for reduction of organic hydroperoxides by organic hydroperoxide reductase (Ohr). LpdA represents the lipoylated disulfide-based enzymes that reduce oxidized Ohr.
Fig. 10
Mechanism of H2O2 degradation by cytochrome
c
peroxidase. The active form of enzyme (Fe2+/Fe3+) reduces H2O2 and forms a Fe4+=O species at the LP-heme site. Reduction occurs through two consecutive deliveries of electrons from cytochome
c
to the HP-heme site.
Fig. 11
Rubrerythrins. A. Domain organization. B. Scheme of electron delivery to recycle rubrerythrin (Rbr). NROR: NADH:rubredoxin oxidoreductase.
Fig. 11
Rubrerythrins. A. Domain organization. B. Scheme of electron delivery to recycle rubrerythrin (Rbr). NROR: NADH:rubredoxin oxidoreductase.
Similar articles
- Heme binding and peroxidase activity of a secreted minicatalase.
Mori G, Doniselli N, Faroldi F, Percudani R. Mori G, et al. FEBS Lett. 2016 Dec;590(24):4495-4506. doi: 10.1002/1873-3468.12493. Epub 2016 Nov 28. FEBS Lett. 2016. PMID: 27859138 - Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli.
Seaver LC, Imlay JA. Seaver LC, et al. J Bacteriol. 2001 Dec;183(24):7173-81. doi: 10.1128/JB.183.24.7173-7181.2001. J Bacteriol. 2001. PMID: 11717276 Free PMC article. - NADH peroxidase activity of rubrerythrin.
Coulter ED, Shenvi NV, Kurtz DM Jr. Coulter ED, et al. Biochem Biophys Res Commun. 1999 Feb 16;255(2):317-23. doi: 10.1006/bbrc.1999.0197. Biochem Biophys Res Commun. 1999. PMID: 10049706 - Reduction of hydrogen peroxide in gram-negative bacteria - bacterial peroxidases.
Nóbrega CS, Pauleta SR. Nóbrega CS, et al. Adv Microb Physiol. 2019;74:415-464. doi: 10.1016/bs.ampbs.2019.02.006. Epub 2019 Apr 8. Adv Microb Physiol. 2019. PMID: 31126534 Review. - Fungal catalases: function, phylogenetic origin and structure.
Hansberg W, Salas-Lizana R, Domínguez L. Hansberg W, et al. Arch Biochem Biophys. 2012 Sep 15;525(2):170-80. doi: 10.1016/j.abb.2012.05.014. Epub 2012 Jun 12. Arch Biochem Biophys. 2012. PMID: 22698962 Review.
Cited by
- Mechanisms of group A Streptococcus resistance to reactive oxygen species.
Henningham A, Döhrmann S, Nizet V, Cole JN. Henningham A, et al. FEMS Microbiol Rev. 2015 Jul;39(4):488-508. doi: 10.1093/femsre/fuu009. Epub 2015 Feb 10. FEMS Microbiol Rev. 2015. PMID: 25670736 Free PMC article. Review. - Oxidoreductases and Reactive Oxygen Species in Conversion of Lignocellulosic Biomass.
Bissaro B, Várnai A, Røhr ÅK, Eijsink VGH. Bissaro B, et al. Microbiol Mol Biol Rev. 2018 Sep 26;82(4):e00029-18. doi: 10.1128/MMBR.00029-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30257993 Free PMC article. Review. - Reserve Flux Capacity in the Pentose Phosphate Pathway by NADPH Binding Is Conserved across Kingdoms.
Christodoulou D, Kuehne A, Estermann A, Fuhrer T, Lang P, Sauer U. Christodoulou D, et al. iScience. 2019 Sep 27;19:1133-1144. doi: 10.1016/j.isci.2019.08.047. Epub 2019 Aug 29. iScience. 2019. PMID: 31536961 Free PMC article. - Adaptive evolution reveals a tradeoff between growth rate and oxidative stress during naphthoquinone-based aerobic respiration.
Anand A, Chen K, Yang L, Sastry AV, Olson CA, Poudel S, Seif Y, Hefner Y, Phaneuf PV, Xu S, Szubin R, Feist AM, Palsson BO. Anand A, et al. Proc Natl Acad Sci U S A. 2019 Dec 10;116(50):25287-25292. doi: 10.1073/pnas.1909987116. Epub 2019 Nov 25. Proc Natl Acad Sci U S A. 2019. PMID: 31767748 Free PMC article. - Resistance to oxidative stress by inner membrane protein ElaB is regulated by OxyR and RpoS.
Guo Y, Li Y, Zhan W, Wood TK, Wang X. Guo Y, et al. Microb Biotechnol. 2019 Mar;12(2):392-404. doi: 10.1111/1751-7915.13369. Epub 2019 Jan 17. Microb Biotechnol. 2019. PMID: 30656833 Free PMC article.
References
- Loew O. Science. 1900;11:701–702. - PubMed
- Naqui A, Chance B. Ann Rev Biochem. 1986;55:137–166. - PubMed
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA. Biochem. Biophys. Res. Commun. 1969;36:891–897. - PubMed
- Messner KR, Imlay JA. J. Biol. Chem. 1999;274:10119–10128. - PubMed
- Messner KR, Imlay JA. J. Biol. Chem. 2002;277:42563–42571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases