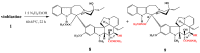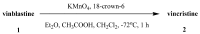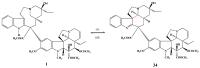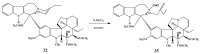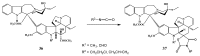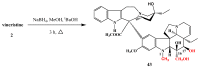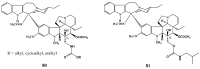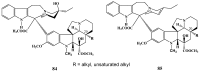Modifications on the basic skeletons of vinblastine and vincristine - PubMed (original) (raw)
Review
Modifications on the basic skeletons of vinblastine and vincristine
Péter Keglevich et al. Molecules. 2012.
Abstract
The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties.
Figures
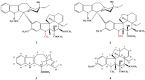
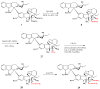
Figure 1
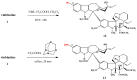
Structures of vinblastine (1) and vincristine (2).
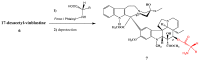
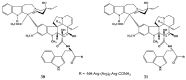
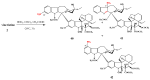
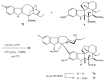
Scheme 1
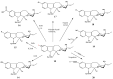
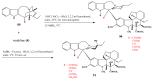
Preparation of 17-desacetylvinblastine-16-amide (5).
Scheme 2
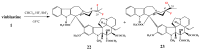
Selective desacetylation of vinblastine (1).
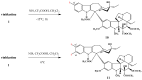
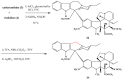
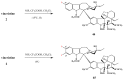
Scheme 3
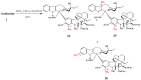
Amino acid derivatives of vinblastine.
Scheme 4
Hydrazide derivatives 8 and 9 of vinblastine (1).
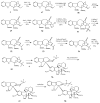
Scheme 5
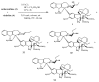
Iodination reactions on vinblastine (1).
Scheme 6
Bromination and formylation of vinblastine (1).
Scheme 7
Reactions of 12′-iodovinblastine (10).
Scheme 8
Reduction of vinblastine (1).
Scheme 9
Fluorination reaction of vinblastine (1).
Scheme 10
Nitration of vinblastine (1).
Scheme 11
Coupling of vinblastine (1) with amino acids.
Scheme 12
Vinblastine derivatives conjugated with carrier peptide.
Scheme 13
Synthesis of vinorelbine (33).
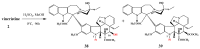
Scheme 14
Oxidation of vinblastine (1) to vincristine (2).
Scheme 15
Ring transformation reaction.
Scheme 16
Preparation of vinamidine (35).
Scheme 17
Spiro-substituted dimer alkaloids.
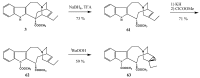
Scheme 18
Deacetylation of vincristine (2).
Scheme 19
Reduction of vincristine (2).
Scheme 20
Nitration of vincristine (2).
Scheme 21
Iodinated derivatives of vincristine (2).
Scheme 22
Selective deacetylation of vincristine (2).
Scheme 23
Detailed synthesis of vinflunine (58).
Scheme 24
Preparation and reaction of 9-chlorocatharanthine (59).
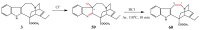
Scheme 25
Building in an oxirane ring to catharanthine (3).
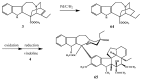
Scheme 26
Preparation of leurosidine (65).
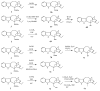
Scheme 27
New derivatives of catharanthine.
Scheme 28
Fluoro-substituted dimeric alkaloids.
Scheme 29
Coupling with saturated vindoline.
Scheme 30
Carbamates of vinblastine.
Scheme 31
Amide substitued anhydrovinblastine derivatives.
Scheme 32
Modification of C-20 ethyl substitutent of vindoline.
Scheme 33
Vinblastines substituted at the vindoline D-ring.
Scheme 34
Coupling vindoline (4) with substituted catharanthines.
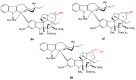
Scheme 35
Coupling in a one-step reaction.
Similar articles
- Synthesis and SAR of vinca alkaloid analogues.
Voss ME, Ralph JM, Xie D, Manning DD, Chen X, Frank AJ, Leyhane AJ, Liu L, Stevens JM, Budde C, Surman MD, Friedrich T, Peace D, Scott IL, Wolf M, Johnson R. Voss ME, et al. Bioorg Med Chem Lett. 2009 Feb 15;19(4):1245-9. doi: 10.1016/j.bmcl.2008.12.077. Epub 2008 Dec 25. Bioorg Med Chem Lett. 2009. PMID: 19147348 - [New antitumor derivatives of vinblastine].
Bölcskei H, Szántay C Jr, Mák M, Balázs M, Szántay C. Bölcskei H, et al. Acta Pharm Hung. 1998 Mar;68(2):87-93. Acta Pharm Hung. 1998. PMID: 9592933 Hungarian. - Studies on the total synthesis of bisindole alkaloids in the vinblastine-vincristine series.
Kutney JP. Kutney JP. Lloydia. 1977 Jan-Feb;40(1):107-26. doi: 10.1002/chin.197711357. Lloydia. 1977. PMID: 875638 - Biotechnological advancements in Catharanthus roseus (L.) G. Don.
Das A, Sarkar S, Bhattacharyya S, Gantait S. Das A, et al. Appl Microbiol Biotechnol. 2020 Jun;104(11):4811-4835. doi: 10.1007/s00253-020-10592-1. Epub 2020 Apr 17. Appl Microbiol Biotechnol. 2020. PMID: 32303816 Review.
Cited by
- Synthesis and Cytotoxic Activity of New Vindoline Derivatives Coupled to Natural and Synthetic Pharmacophores.
Keglevich A, Dányi L, Rieder A, Horváth D, Szigetvári Á, Dékány M, Szántay C Jr, Latif AD, Hunyadi A, Zupkó I, Keglevich P, Hazai L. Keglevich A, et al. Molecules. 2020 Feb 24;25(4):1010. doi: 10.3390/molecules25041010. Molecules. 2020. PMID: 32102414 Free PMC article. - A Combined Molecular Docking/Dynamics Approach to Probe the Binding Mode of Cancer Drugs with Cytochrome P450 3A4.
Panneerselvam S, Yesudhas D, Durai P, Anwar MA, Gosu V, Choi S. Panneerselvam S, et al. Molecules. 2015 Aug 14;20(8):14915-35. doi: 10.3390/molecules200814915. Molecules. 2015. PMID: 26287147 Free PMC article. - Enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation.
Liu J, Zhu J, Tang L, Wen W, Lv S, Yu R. Liu J, et al. World J Microbiol Biotechnol. 2014 Jan;30(1):175-80. doi: 10.1007/s11274-013-1432-z. Epub 2013 Jul 18. World J Microbiol Biotechnol. 2014. PMID: 23864440 - An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole.
Payne RM, Xu D, Foureau E, Teto Carqueijeiro MI, Oudin A, Bernonville TD, Novak V, Burow M, Olsen CE, Jones DM, Tatsis EC, Pendle A, Ann Halkier B, Geu-Flores F, Courdavault V, Nour-Eldin HH, O'Connor SE. Payne RM, et al. Nat Plants. 2017 Jan 13;3:16208. doi: 10.1038/nplants.2016.208. Nat Plants. 2017. PMID: 28085153 Free PMC article. - Exploring the potential of two Pseudomonas species to produce vincristine from vinblastine via biotransformation.
Srivastava G, Mittal R, Srivastava N, Ganjewala D. Srivastava G, et al. Sci Rep. 2024 Aug 23;14(1):19652. doi: 10.1038/s41598-024-70571-8. Sci Rep. 2024. PMID: 39179785 Free PMC article.
References
- Brossi A., Suffness M. The Alkaloids. Vol. 37. Academic Press Inc.; New York, NY, USA: 1990. Antitumor Bisindol Alkaloids from Catharanthus Roseus (L.) pp. 1–240.
- Bölcskei H., Szabó L., Szántay C. Synthesis of vinblastine derivatives. Front. Nat. Prod. Chem. 2005;1:43–49. doi: 10.2174/1574089054583849. - DOI
- Eli Lilly Company, Neue Aminderivate von Vinblastin, Leurosidin und Leurocristin und Verfahren zu ihrer Herstellung. 22415980. DE Patent. 1974 [Chem. Abstr. 1974, 82, 579967b]
- Barnett C.J., Cullinan G.J., Gerzon K., Hoying R.C., Jones W.E., Newlon W.M., Poore G.A., Robison R.L., Sweeney M.J., Todd G.C. Structure-activity relationships of dimeric Catharanthus alkaloids. 1. Deacetylvinblastine amide (vindesine) sulfate. J. Med. Chem. 1978;21:88–96. - PubMed
- Thimmaiah K.N., Lloyd W.D., Sethi V.S. A simple method for the chemical modification of antitumor Catharanthus Vinca alkaloids. Ind. J. Chem. Sect. B: Org. Chem. Med. Chem. 1990;29:678–680.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources