Synergistic activity of the mTOR inhibitor ridaforolimus and the antiandrogen bicalutamide in prostate cancer models - PubMed (original) (raw)
Synergistic activity of the mTOR inhibitor ridaforolimus and the antiandrogen bicalutamide in prostate cancer models
Rachel M Squillace et al. Int J Oncol. 2012 Aug.
Abstract
Although androgen ablation therapy is the foundation of current prostate cancer treatment, most patients ultimately develop castration-resistant disease. One proposed mechanism to account for androgen receptor (AR) activity in the castrate environment is via crosstalk with other signaling pathways. Specifically, reciprocal interactions between the AKT/mTOR and AR pathways have been implicated in prostate cancer progression. Here, we used the potent inhibitor ridaforolimus to target mTOR signaling alone and in combination with AR blockade by bicalutamide to examine the effect of abrogating these signaling pathways. Ridaforolimus treatment inhibited the proliferation of all six prostate cancer cell lines examined with the greatest sensitivity associated with loss of PTEN and elevated AKT/mTOR pathway activity. Dual inhibition of the AR and mTOR signaling pathways provided further benefit with the ridaforolimus-bicalutamide combination producing synergistic antiproliferative effects in prostate cancer cells in vitro when compared with each agent alone. Pharmacodynamic analysis confirmed that combination treatment resulted in full inhibition of each of the respective pathways. Importantly, the ridaforolimus-bicalutamide combination exhibited potent antitumor activity with parallel reductions in plasma PSA levels in vivo. Taken together, ridaforolimus exhibited potent antiproliferative and antitumor activity in prostate cancer models and the addition of bicalutamide represents a potentially effective combination strategy for patient therapy.
Figures
Figure 1
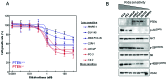
PTEN loss and elevated AKT/mTOR activity is associated with ridaforolimus sensitivity in prostate cell lines. To evaluate the level of sensitivity of cells to ridaforolimus we measured the effect on the rate of cell proliferation, rather than on the absolute cell number since the effect of a cytostatic drug on absolute cell number is directly influenced by the intrinsic cell doubling time. (A) Determination of ridaforolimus sensitivity in a panel of prostate cancer as well as prostate epithelial (RWPE-1) cell lines cultured with ridaforolimus over a 0.0001–1,000 nM concentration range for 3 days. PTEN wild-type cells (PTEN+/+) are shown in blue and PTEN null (PTEN−/−) shown in red. (B) Relationship between PTEN/AKT/mTOR pathway markers and ridaforolimus sensitivity. Cell lines are presented with decreasing sensitivity to ridaforolimus, as determined in (A). Cellular extracts from the prostate cell lines were prepared and equal amounts of total protein were analyzed by immunoblotting for PTEN, phospho-AKT (Ser473), AKT, phospho-S6 (Ser235/236), ribosomal protein S6, phospho-4EBP1 (Ser65/Thr70) and VDAC (as a loading control).
Figure 2
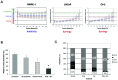
Simultaneous AR and mTOR pathway blockade results in synergistic growth inhibition in prostate cancer lines. (A) RWPE-1, LNCaP and C4-2 cell lines were treated with increasing concentrations of ridaforolimus, bicalutamide, or both, and the effects on proliferation determined. The Combination Index (CI) was calculated using Median Effect analysis. Strict criteria were applied to drug interaction analysis, where synergy was defined as CI <0.75, additivity as >0.75 CI <1.25, and antagonism as CI >1.25. Data expressed as mean CI (± SD), determined for a range of drug concentrations and a fractional effect (Fa) of 0.2 to 0.8 over the complete dosing range. (B) Soft agar clonogenic assay to determine the effects of the ridaforolimus and bicalutamide combination on anchorage-independent growth of C4-2 prostate cancer cells. C4-2 cells were treated with medium alone, bicalutamide (10 _μ_M), ridaforolimus (0.5 nM) or the combination (Rida + Bic) for 2 weeks. The percentage colony formation (compared to untreated controls) are presented as means ± SD for duplicate experiments. *p-value ≤0.01 compared with vehicle treated cells. (C) Ridaforolimus and bicalutamide in combination induce cell cycle arrest in prostate cancer cells. C4-2 prostate cancer cells were treated with vehicle, ridaforolimus (Rida; 50 nM), bicalutamide (Bic; 50 _μ_M) or the combination (Rida + Bic) for 24 h. Cells were harvested, stained with propidium iodide and analyzed by flow cytometry to determine DNA content. The percentage of cells in G1, S or G2/M phase was calculated from FL-2 histograms using ModFit Lt software. *p-value ≤0.05 compared with single agents or vehicle treated cells.
Figure 3
Combination of ridaforolimus plus bicalutamide inhibits AR and mTOR signaling; PSA levels mirror cell growth in combination-treated prostate cell lines. (A) In the left panel RWPE-1, LNCaP and C4-2 cells were treated for 24 h with vehicle alone (Ǿ), 10 _μ_M bicalutamide (B), 0.5 nM ridaforolimus (R), or the combination (B+R). In the right panel, RWPE-1, LNCaP and C4-2 cells were treated with the combination of ridaforolimus and bicalutamide for up to 3 days with lysates harvested at the indicated times. Cellular extracts were immunoblotted for AR, PSA, phospho-S6 (Ser235/236), ribosomal protein S6 and VDAC (as a control). (B) LNCaP and C4-2 cells were treated with ridaforolimus as a single agent at the indicated concentrations (upper panels), or with the ridaforolimus/bicalutamide combination at the range of concentrations indicated (lower panels) for 3 days. PSA levels in the supernatant were determined by ELISA and relative secretion presented as the ratio of compound- versus vehicle-treated cells. The effects on proliferation were evaluated and cell growth shown as a percentage of vehicle controls. Data presented as means of ≥3 individual experiments ± SD.
Figure 4
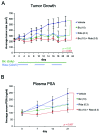
Ridaforolimus and bicalutamide combination induces potent anti-tumor activity with corresponding PSA level reduction in vivo. Mice bearing C4-2 prostate cancer xenografts (200 mm3) were randomized into four groups (n=10 mice/group). Mice were treated with either vehicle, bicalutamide (Bic; 10 mg/kg), ridaforolimus (Rid; 0.3 mg/kg) or the combination. Ridaforolimus was administered daily for 5 days followed by a 2 day break (QDx5), and bicalutamide administered daily. Three cycles of dosing were completed (21 days). (A) Mean tumor volumes were calculated using caliper measurements and data are plotted as mean ± SE over treatment time. (B) Blood was harvested from the animals on days 0, 7, 14 and 21 and plasma PSA levels determined by ELISA. Data are presented as mean serum concentrations (ng/ml) ± SE for each treatment group.
Similar articles
- Prolonging hormone sensitivity in prostate cancer xenografts through dual inhibition of AR and mTOR.
Schayowitz A, Sabnis G, Goloubeva O, Njar VC, Brodie AM. Schayowitz A, et al. Br J Cancer. 2010 Sep 28;103(7):1001-7. doi: 10.1038/sj.bjc.6605882. Epub 2010 Sep 14. Br J Cancer. 2010. PMID: 20842117 Free PMC article. - The androgen receptor is a negative regulator of eIF4E phosphorylation at S209: implications for the use of mTOR inhibitors in advanced prostate cancer.
D'Abronzo LS, Bose S, Crapuchettes ME, Beggs RE, Vinall RL, Tepper CG, Siddiqui S, Mudryj M, Melgoza FU, Durbin-Johnson BP, deVere White RW, Ghosh PM. D'Abronzo LS, et al. Oncogene. 2017 Nov 16;36(46):6359-6373. doi: 10.1038/onc.2017.233. Epub 2017 Jul 24. Oncogene. 2017. PMID: 28745319 Free PMC article. - Tolerability, safety and pharmacokinetics of ridaforolimus in combination with bicalutamide in patients with asymptomatic, metastatic castration-resistant prostate cancer (CRPC).
Meulenbeld HJ, de Bono JS, Tagawa ST, Whang YE, Li X, Heath KH, Zandvliet AS, Ebbinghaus SW, Hudes GR, de Wit R. Meulenbeld HJ, et al. Cancer Chemother Pharmacol. 2013 Oct;72(4):909-16. doi: 10.1007/s00280-013-2250-6. Epub 2013 Aug 7. Cancer Chemother Pharmacol. 2013. PMID: 23921574 Clinical Trial. - Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer.
Pungsrinont T, Kallenbach J, Baniahmad A. Pungsrinont T, et al. Int J Mol Sci. 2021 Oct 14;22(20):11088. doi: 10.3390/ijms222011088. Int J Mol Sci. 2021. PMID: 34681745 Free PMC article. Review. - Clinical research progress of ridaforolimus (AP23573, MK8668) over the past decade: a systemic review.
Wang L, Qiu Q, Yang D, Cao C, Lu Y, Zeng Y, Jiang W, Shen Y, Ye Y. Wang L, et al. Front Pharmacol. 2024 Mar 22;15:1173240. doi: 10.3389/fphar.2024.1173240. eCollection 2024. Front Pharmacol. 2024. PMID: 38584599 Free PMC article. Review.
Cited by
- Evidence of mTOR Activation by an AKT-Independent Mechanism Provides Support for the Combined Treatment of PTEN-Deficient Prostate Tumors with mTOR and AKT Inhibitors.
Zhang W, Haines BB, Efferson C, Zhu J, Ware C, Kunii K, Tammam J, Angagaw M, Hinton MC, Keilhack H, Paweletz CP, Zhang T, Winter C, Sathyanarayanan S, Cheng J, Zawel L, Fawell S, Gilliland G, Majumder PK. Zhang W, et al. Transl Oncol. 2012 Dec;5(6):422-9. doi: 10.1593/tlo.12241. Epub 2012 Dec 1. Transl Oncol. 2012. PMID: 23323157 Free PMC article. - Hydralazine and Enzalutamide: Synergistic Partners against Prostate Cancer.
Lopes N, Pacheco MB, Soares-Fernandes D, Correia MP, Camilo V, Henrique R, Jerónimo C. Lopes N, et al. Biomedicines. 2021 Aug 7;9(8):976. doi: 10.3390/biomedicines9080976. Biomedicines. 2021. PMID: 34440180 Free PMC article. - Lemur Tyrosine Kinase 2, a novel target in prostate cancer therapy.
Shah K, Bradbury NA. Shah K, et al. Oncotarget. 2015 Jun 10;6(16):14233-46. doi: 10.18632/oncotarget.3899. Oncotarget. 2015. PMID: 26008968 Free PMC article. - Combinatorial Effect of Abiraterone Acetate and NVP-BEZ235 on Prostate Tumor Progression in Rats.
Gonçalves BF, de Campos SGP, Fávaro WJ, Brandt JZ, Pinho CF, Justulin LA, Taboga SR, Scarano WR. Gonçalves BF, et al. Horm Cancer. 2018 Jun;9(3):175-187. doi: 10.1007/s12672-018-0323-z. Epub 2018 Jan 23. Horm Cancer. 2018. PMID: 29363091 Free PMC article. - Noninvasive Measurement of mTORC1 Signaling with 89Zr-Transferrin.
Truillet C, Cunningham JT, Parker MFL, Huynh LT, Conn CS, Ruggero D, Lewis JS, Evans MJ. Truillet C, et al. Clin Cancer Res. 2017 Jun 15;23(12):3045-3052. doi: 10.1158/1078-0432.CCR-16-2448. Epub 2016 Dec 22. Clin Cancer Res. 2017. PMID: 28007777 Free PMC article.
References
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142–148. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous