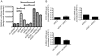Multiple interferon stimulated genes synergize with the zinc finger antiviral protein to mediate anti-alphavirus activity - PubMed (original) (raw)
Multiple interferon stimulated genes synergize with the zinc finger antiviral protein to mediate anti-alphavirus activity
Sophiya Karki et al. PLoS One. 2012.
Abstract
The zinc finger antiviral protein (ZAP) is a host factor that mediates inhibition of viruses in the Filoviridae, Retroviridae and Togaviridae families. We previously demonstrated that ZAP blocks replication of Sindbis virus (SINV), the prototype Alphavirus in the Togaviridae family at an early step prior to translation of the incoming genome and that synergy between ZAP and one or more interferon stimulated genes (ISGs) resulted in maximal inhibitory activity. The present study aimed to identify those ISGs that synergize with ZAP to mediate Alphavirus inhibition. Using a library of lentiviruses individually expressing more than 350 ISGs, we screened for inhibitory activity in interferon defective cells with or without ZAP overexpression. Confirmatory tests of the 23 ISGs demonstrating the largest infection reduction in combination with ZAP revealed that 16 were synergistic. Confirmatory tests of all potentially synergistic ISGs revealed 15 additional ISGs with a statistically significant synergistic effect in combination with ZAP. These 31 ISGs are candidates for further mechanistic studies. The number and diversity of the identified ZAP-synergistic ISGs lead us to speculate that ZAP may play an important role in priming the cell for optimal ISG function.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
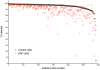
Figure 1. Anti-SINV activity of a library of 383 ISGs in control and ZAP-expressing cells.
BHK/HA-Zeo (Control) cells or BHK/NZAP-Zeo cells expressing the amino terminal domain of rat ZAP (ZAP cells) were transduced with lentiviruses co-expressing individual ISGs and the red fluorescent protein TagRFP. After 2 d, the cells were challenged with SINV expressing GFP (moi = 5). After 8 h, the cells were harvested and analyzed by flow cytometry to determine the percentage of infected cells (GFP+) within the transduced (RFP+) population. Red symbols indicate cells expressing the control protein, Fluc, while black open circles indicate cells expressing the individual ISGs. For each cell type, the line in the scatter plot indicates the mean value for the percentage of infected cells. Gene symbols are shown for ISGs resulting in infection rates below an arbitrary cutoff of 85% (dashed line).
Figure 2. Comparison of the antiviral activity of 292 ISGs in the control cells versus ZAP-expressing cells.
For those 292 ISGs with infection data in each cell type the results were sorted based on the percentage of infected cells in the control cells and the paired results are plotted versus an arbitrary ISG number. For each ISG, black circles show the % infected in the control cells while red circles show the % infected in the ZAP cells. Results obtained in the absence of ISG expression (Fluc) are shown with the filled circles.
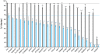
Figure 3. Reduction in the percentage of infected cells by ZAP.
For each ISG the reduction in the percentage of infected cells due to ZAP co-expression was calculated by subtracting the percentage of infected cells in the ZAP cells from the percentage infected in the control cells. After sorting, the differences were plotted versus an arbitrary ISG number. The difference seen between the control and ZAP cells in the absence of ISG expression (Fluc) is shown by the red symbol. Gene symbols are shown for the 23 ISGs with the greatest difference in infection percentage (≥18) due to ZAP expression.
Figure 4. Confirmatory testing of the top ISG hits synergizing with ZAP.
Triplicate wells of BHK/HA-Zeo cells (Control cells, gray bars) or BHK/NZAP-Zeo cells expressing the amino terminal domain of rat ZAP (ZAP cells, blue bars) were transduced with lentiviruses co-expressing the indicated ISGs and the red fluorescent protein TagRFP. After 2 d, the cells were challenged with SINV expressing GFP (moi = 5). After 8 h, the cells were harvested and analyzed by flow cytometry to determine the percentage of infected cells (GFP+) within the transduced (RFP+) population. Mean values are plotted; error bars indicate the standard deviation. Dashed lines indicate the percentage of infection determined in control cells expressing Fluc (gray) or ZAP cells expressing Fluc (blue). For FLJ39739 transduction of ZAP cells, there was only one replicate for analysis. Asterisks indicate mean values statistically different than values obtained in Fluc-expressing cells for the corresponding cell type (unpaired t test, *, P<0.05; **, P<0.01; ***, P<0.001).
Figure 5. Validation of synergy between ZAP and the top three ISGs in a knockdown system.
A) Triplicate wells of Huh-7 cells were transfected with irrelevant siRNA, ZAP-specific siRNA, ISG-specific siRNA that targets IRF2, RIG-I or IL28RA, or siRNAs that target both ZAP and an ISG. ISG-specific siRNA was added to cells again on the second day after seeding. Forty-eight h after initial siRNA transfection, cells were infected with Toto1101/Luc (moi = 5). Viral replication was determined by firefly luciferase activity 4 h after infection. Huh-7 cells that were not transfected with siRNA were included as a negative control. Means and standard deviations of triplicate samples are shown. Asterisks indicate mean values statistically different between two siRNA treatments (unpaired t test, *, P<0.05; **, P<0.01; ***, P<0.001). B) Forty-eight h after initial siRNA transfection, total RNA was extracted from the cells and used to generate cDNA. RNA levels of IRF2, RIG-I, IL28RA and RPS11 were measured by real-time PCR. The ISG mRNA levels were normalized with that of RPS11, and the ISG mRNA levels in irrelevant siRNA-transfected cells were set as 1. Data are means +/− SD of one experiment in triplicate.
Similar articles
- The zinc finger antiviral protein acts synergistically with an interferon-induced factor for maximal activity against alphaviruses.
MacDonald MR, Machlin ES, Albin OR, Levy DE. MacDonald MR, et al. J Virol. 2007 Dec;81(24):13509-18. doi: 10.1128/JVI.00402-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928353 Free PMC article. - Expression of the zinc-finger antiviral protein inhibits alphavirus replication.
Bick MJ, Carroll JW, Gao G, Goff SP, Rice CM, MacDonald MR. Bick MJ, et al. J Virol. 2003 Nov;77(21):11555-62. doi: 10.1128/jvi.77.21.11555-11562.2003. J Virol. 2003. PMID: 14557641 Free PMC article. - Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions.
Law LM, Albin OR, Carroll JW, Jones CT, Rice CM, Macdonald MR. Law LM, et al. J Virol. 2010 May;84(9):4504-12. doi: 10.1128/JVI.02018-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181706 Free PMC article. - Interferon-Stimulated Genes that Target Retrovirus Translation.
Jäger N, Pöhlmann S, Rodnina MV, Ayyub SA. Jäger N, et al. Viruses. 2024 Jun 8;16(6):933. doi: 10.3390/v16060933. Viruses. 2024. PMID: 38932225 Free PMC article. Review. - ADAR1 and PKR, interferon stimulated genes with clashing effects on HIV-1 replication.
Radetskyy R, Daher A, Gatignol A. Radetskyy R, et al. Cytokine Growth Factor Rev. 2018 Apr;40:48-58. doi: 10.1016/j.cytogfr.2018.03.007. Epub 2018 Mar 26. Cytokine Growth Factor Rev. 2018. PMID: 29625900 Review.
Cited by
- TRIM32 inhibits Venezuelan equine encephalitis virus infection by targeting a late step in viral entry.
Xie Y, Cao J, Gan S, Xu L, Zhang D, Qian S, Xu F, Ding Q, Schoggins JW, Fan W. Xie Y, et al. PLoS Pathog. 2024 Nov 11;20(11):e1012312. doi: 10.1371/journal.ppat.1012312. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39527628 Free PMC article. - Versatility of the Zinc-Finger Antiviral Protein (ZAP) As a Modulator of Viral Infections.
Shao R, Visser I, Fros JJ, Yin X. Shao R, et al. Int J Biol Sci. 2024 Aug 26;20(12):4585-4600. doi: 10.7150/ijbs.98029. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309436 Free PMC article. Review. - TRIM32 inhibits Venezuelan Equine Encephalitis Virus Infection by targeting a late step in viral entry.
Xie Y, Cao J, Gan S, Xu L, Zhang D, Qian S, Xu F, Ding Q, Schoggins JW, Fan W. Xie Y, et al. bioRxiv [Preprint]. 2024 Jun 4:2024.06.04.597282. doi: 10.1101/2024.06.04.597282. bioRxiv. 2024. PMID: 38895352 Free PMC article. Updated. Preprint. - Single-cell analysis reveals an antiviral network that controls Zika virus infection in human dendritic cells.
Moore KM, Pelletier A-N, Lapp S, Metz A, Tharp GK, Lee M, Bhasin SS, Bhasin M, Sékaly R-P, Bosinger SE, Suthar MS. Moore KM, et al. J Virol. 2024 May 14;98(5):e0019424. doi: 10.1128/jvi.00194-24. Epub 2024 Apr 3. J Virol. 2024. PMID: 38567950 Free PMC article. - Transcriptome regulation by PARP13 in basal and antiviral states in human cells.
Busa VF, Ando Y, Aigner S, Yee BA, Yeo GW, Leung AKL. Busa VF, et al. iScience. 2024 Feb 16;27(4):109251. doi: 10.1016/j.isci.2024.109251. eCollection 2024 Apr 19. iScience. 2024. PMID: 38495826 Free PMC article.
References
- Griffin DE. Alphaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1023–1067.
- Gao G, Guo X, Goff SP. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science. 2002;297:1703–1706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54-AI057158/AI/NIAID NIH HHS/United States
- R01 AI091707/AI/NIAID NIH HHS/United States
- UL1 RR024143/RR/NCRR NIH HHS/United States
- AI057905/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- R01 AI057905/AI/NIAID NIH HHS/United States
- AI091707/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources