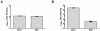18β-glycyrrhetinic acid inhibits rotavirus replication in culture - PubMed (original) (raw)
18β-glycyrrhetinic acid inhibits rotavirus replication in culture
Michele E Hardy et al. Virol J. 2012.
Abstract
Background: Glycyrrhizin (GA) and primary metabolite 18β-glycyrrhetinic acid (GRA) are pharmacologically active components of the medicinal licorice root, and both have been shown to have antiviral and immunomodulatory properties. Although these properties are well established, the mechanisms of action are not completely understood. In this study, GA and GRA were tested for the ability to inhibit rotavirus replication in cell culture, toward a long term goal of discovering natural compounds that may complement existing vaccines.
Methods: Epithelial cells were treated with GA or GRA various times pre- or post-infection and virus yields were measured by immunofluorescent focus assay. Levels of viral proteins VP2, VP6, and NSP2 in GRA treated cells were measured by immunoblot to determine if there was an effect of GRA treatment on the accumulation of viral protein.
Results: GRA treatment reduced rotavirus yields by 99% when added to infected cultures post-- virus adsorption, whereas virus yields in GA treated cultures were similar to mock treated controls. Time of addition experiments indicated that GRA-mediated replication inhibition likely occurs at a step or steps subsequent to virus entry. The amounts of VP2, VP6 and NSP2 were substantially reduced when GRA was added to cultures up to two hours post-entry.
Conclusions: GRA, but not GA, has significant antiviral activity against rotavirus replication in vitro, and studies to determine whether GRA attenuates rotavirus replication in vivo are underway.
Figures
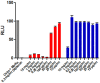
Figure 1
GRA and GA cytotoxicity in MA104 cells MA104 cells were treated for six hours with the indicated concentrations of GA or GRA. Viability was measured with the Promega CellTiterGlo Assay according to the manufacturer’s protocol, with digitonin as the control for 100% cytotoxicity. Red bars indicate GRA and blue bars GA. Data shown are representative of two experiments, with each concentration tested in triplicate in each experiment. Error bars indicate SEM.
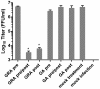
Figure 2
GRA reduces rotavirus yields when added post--infection. MA104 cells were infected with rotavirus strain RRV at a multiplicity of infection of 3 pfu/cell. 25 μg/mL GRA was added to the cultures either six hours pre-infection and then removed for the remaining time (pre) or was present continuously in the media throughout the course of infection (pre/post). For post-infection treatments, GRA was added to the cultures following the one hour virus adsorption period and maintained in the media throughout the infection. Total virus was harvested from cells and supernatant, and yields of infectious virus were determined by IFA. Data shown are representative of two experiments with triplicate samples in each experiment. Analysis for significance was performed using a two--‒tailed Student’s t test. Errors indicate SEM. *p = 0.006.
Figure 3
GRA does not inactivate the rotavirus particle and inhibits replication postentry. A) RRV stocks were incubated with 25 μg/mL GRA or DMSO for one hour at 37°C. Virus then was concentrated by ultracentrifugation for two hours at 35,000 rpm in an SW55 rotor (Beckman) and infectivity was measured by IFA. Data are from triplicate samples, p < 0.001. B) Trypsin‒activated RRV was inoculated onto cells at a multiplicity of infection of 3 pfu/cell and adsorbed for one hour at 4°C. Following adsorption, the medium was replaced and cultures were incubated for one hour at 37°C to allow virus entry. 25 μg/mL GRA or DMSO was added to the culture following the 37°C incubation, and virus titers following an additional 5 hours of infection were determined by IFA. Data were analyzed for significance with the two‒tailed Student’s t test. Error bars indicate SEM. p < 0.004.
Figure 4
GRA treatment reduces the levels of VP2, VP6 and NSP2 in infected cells. Cells were inoculated with RRV at a multiplicity of infection of 3 pfu/cell. Virus was adsorbed for one hour at 4°C, and cultures then were shifted to 37°C for one hour (A) or two hours (B) prior to addition of GRA. Lysates were probed in immunoblots with anti-rotavirus strain SA11F antiserum to detect VP2 and VP6, anti‒NSP2, or anti‒GAPDH as a loading control. The lanes are triplicate samples. Bands were quantified by densitometry taking the averaged intensity of the bands in the individual samples normalized to the averaged intensity of the GAPDH loading control in each sample.
Similar articles
- 18β-glycyrrhetinic acid delivered orally induces isolated lymphoid follicle maturation at the intestinal mucosa and attenuates rotavirus shedding.
Hendricks JM, Hoffman C, Pascual DW, Hardy ME. Hendricks JM, et al. PLoS One. 2012;7(11):e49491. doi: 10.1371/journal.pone.0049491. Epub 2012 Nov 13. PLoS One. 2012. PMID: 23152913 Free PMC article. - 18β-Glycyrrhetinic acid inhibits the apoptosis of cells infected with rotavirus SA11 via the Fas/FasL pathway.
Wang X, Xie F, Zhou X, Chen T, Xue Y, Wang W. Wang X, et al. Pharm Biol. 2021 Dec;59(1):1098-1105. doi: 10.1080/13880209.2021.1961821. Pharm Biol. 2021. PMID: 34411493 Free PMC article. Retracted. - Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro.
Tohmé MJ, Giménez MC, Peralta A, Colombo MI, Delgui LR. Tohmé MJ, et al. Int J Antimicrob Agents. 2019 Nov;54(5):601-609. doi: 10.1016/j.ijantimicag.2019.07.015. Epub 2019 Jul 26. Int J Antimicrob Agents. 2019. PMID: 31356859 - Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae.
Taraporewala ZF, Patton JT. Taraporewala ZF, et al. Virus Res. 2004 Apr;101(1):57-66. doi: 10.1016/j.virusres.2003.12.006. Virus Res. 2004. PMID: 15010217 Review. - Can Antiviral Activity of Licorice Help Fight COVID-19 Infection?
Diomede L, Beeg M, Gamba A, Fumagalli O, Gobbi M, Salmona M. Diomede L, et al. Biomolecules. 2021 Jun 8;11(6):855. doi: 10.3390/biom11060855. Biomolecules. 2021. PMID: 34201172 Free PMC article. Review.
Cited by
- Anti-dengue viral activity of Glycyrrhiza glabra roots in Vero cells.
Jayasekara KG, Suresh S, Goonasekara C, Soyza P, Perera N, Gunasekera K. Jayasekara KG, et al. Sci Rep. 2024 Oct 29;14(1):25922. doi: 10.1038/s41598-024-76184-5. Sci Rep. 2024. PMID: 39472523 Free PMC article. - Application of 18β-glycyrrhetinic acid in the structural modification of natural products: a review.
Li WX, Lu YF, Wang F, Ai B, Jin SB, Li S, Xu GH, Jin CH. Li WX, et al. Mol Divers. 2024 Apr 29. doi: 10.1007/s11030-024-10864-2. Online ahead of print. Mol Divers. 2024. PMID: 38683490 Review. - A review of typical biological activities of glycyrrhetinic acid and its derivatives.
Chen L, Gong J, Yong X, Li Y, Wang S. Chen L, et al. RSC Adv. 2024 Feb 22;14(10):6557-6597. doi: 10.1039/d3ra08025k. eCollection 2024 Feb 21. RSC Adv. 2024. PMID: 38390501 Free PMC article. Review. - Glycyrrhizinic Acid as an Antiviral and Anticancer Agent in the Treatment of Human Papillomavirus.
Bravo V, Serrano M, Duque A, Ferragud J, Coronado PJ. Bravo V, et al. J Pers Med. 2023 Nov 24;13(12):1639. doi: 10.3390/jpm13121639. J Pers Med. 2023. PMID: 38138866 Free PMC article. Review. - Development of an Antiviral Ion-Activated In Situ Gel Containing 18β-Glycyrrhetinic Acid: A Promising Alternative against Respiratory Syncytial Virus.
Özkan B, Altuntaş E, Ünlü Ü, Doğan HH, Özsoy Y, Çakır Koç R. Özkan B, et al. Pharmaceutics. 2023 Jul 31;15(8):2055. doi: 10.3390/pharmaceutics15082055. Pharmaceutics. 2023. PMID: 37631269 Free PMC article.
References
- Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;Suppl 1:S1–S8. - PubMed
- Ciarlet M, Schodel F. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine. 2009;27 Suppl 6:G72–G81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources