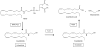The thrifty lipids: endocannabinoids and the neural control of energy conservation - PubMed (original) (raw)
Review
The thrifty lipids: endocannabinoids and the neural control of energy conservation
Nicholas V DiPatrizio et al. Trends Neurosci. 2012 Jul.
Abstract
The 'thrifty gene hypothesis' posits that evolution preferentially selects physiological mechanisms that optimize energy storage to increase survival under alternating conditions of abundance and scarcity of food. Recent experiments suggest that endocannabinoids - a class of lipid-derived mediators that activate cannabinoid receptors in many cells of the body - are key agents of energy conservation. The new evidence indicates that these compounds increase energy intake and decrease energy expenditure by controlling the activity of peripheral and central neural pathways involved in the sensing and hedonic processing of sweet and fatty foods, as well as in the storage of their energy content for future use.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure I
Schematic illustrating the main enzymatic steps involved in the formation and hydrolysis of anandamide.
Figure I
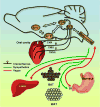
Schematic representing key central and peripheral organs involved in food intake and energy balance. Gustatory neural signals, including those likely associated with fat or sweet taste, are transmitted from the tongue and oral cavity to the brainstem along the facial (CNVII), glossopharyngeal (CNIX), and vagus (CNX) nerves [22]. These afferent sensory signals terminate in the nucleus of the solitary tract (NST). Neural signals are subsequently transmitted rostrally in rats to the parabrachial nucleus (PBN). Neurons in the NST and PBN respond to and integrate gustatory information derived from the oral cavity, with satiation/satiety-related neural signals transmitted from the gut by the afferent vagus nerve (red arrows). The hindbrain communicates sensory information from food to areas throughout the forebrain, including the nucleus accumbens (NAc) and the hypothalamus (HYP). Importantly, the brain communicates with peripheral organs and tissues, including brown adipose tissue (BAT), liver, white adipose tissue (WAT), and small intestine (SI), via the autonomic nervous system —which comprises vagal afferent and efferents (red arrows), and sympathetics (green arrows) — to maintain food intake and energy balance.
Figure I. Endocannabinoid mechanisms are involved in controlling sympathetic activity that drives BAT thermogenesis
(A) Schematic diagram of the rat brain (upper panel) illustrating neural centers in the HYP and BS that control thermogenesis in BAT. Lower panel: Norepinephrine released from sympathetic nerve terminals results in the release of heat from brown adipocytes. (B–C) Electron microscopy revealed increased mitochondrial density in BAT from MGL-transgenic mice (left panel) compared to wild-type mice (right panel). Images adapted from [59]; red asterisk represents mitochondria.
Figure 1
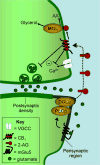
Receptor-dependent production of 2-arachidonoyl-_sn_-glycerol (2-AG) and retrograde signaling at excitatory synapses. The biosynthesis of 2-AG at excitatory synapses may be initiated following spillover of glutamate into the perisynaptic region. Glutamate signaling at type-5 metabotropic glutamate receptors (mGlu5-Rs) stimulates phospholipase C-β (PLC-β) activity generating 1,2-arachidonoylglycerol, which is cleaved by diacylglycerol lipase-α (DGL-α) to produce 2-AG [6, 8, 15]. This endocannabinoid diffuses to the nerve ending where it binds to presynaptic CB1 cannabinoid receptors, reducing both calcium influx at voltage gated calcium channels (VGCC) and vesicular release of glutamate (inhibition denoted by –). 2-AG is rapidly degraded by monoacylglycerol lipase (MGL) and other hydrolases [9] into arachidonic acid (AA) and glycerol.
Similar articles
- Central and peripheral signaling mechanisms involved in endocannabinoid regulation of feeding: a perspective on the munchies.
Sharkey KA, Pittman QJ. Sharkey KA, et al. Sci STKE. 2005 Mar 29;2005(277):pe15. doi: 10.1126/stke.2772005pe15. Sci STKE. 2005. PMID: 15798103 Review. - Endogenous cannabinoids in the brain and peripheral tissues: regulation of their levels and control of food intake.
Matias I, Bisogno T, Di Marzo V. Matias I, et al. Int J Obes (Lond). 2006 Apr;30 Suppl 1:S7-S12. doi: 10.1038/sj.ijo.0803271. Int J Obes (Lond). 2006. PMID: 16570107 Review. - [The endocannabinoid system].
Sudano I, Périat D, Noll G. Sudano I, et al. Praxis (Bern 1994). 2008 Apr 2;97(7):375-80. doi: 10.1024/1661-8157.97.7.375. Praxis (Bern 1994). 2008. PMID: 18548817 German. - Endocannabinoids and the control of energy homeostasis.
Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Bátkai S. Kunos G, et al. J Biol Chem. 2008 Nov 28;283(48):33021-5. doi: 10.1074/jbc.R800012200. Epub 2008 Aug 11. J Biol Chem. 2008. PMID: 18694938 Free PMC article. Review. - Endocannabinoid control of food intake and energy balance.
Di Marzo V, Matias I. Di Marzo V, et al. Nat Neurosci. 2005 May;8(5):585-9. doi: 10.1038/nn1457. Nat Neurosci. 2005. PMID: 15856067 Review.
Cited by
- Links between central CB1-receptor availability and peripheral endocannabinoids in patients with first episode psychosis.
Dickens AM, Borgan F, Laurikainen H, Lamichhane S, Marques T, Rönkkö T, Veronese M, Lindeman T, Hyötyläinen T, Howes O, Hietala J, Orešič M. Dickens AM, et al. NPJ Schizophr. 2020 Aug 26;6(1):21. doi: 10.1038/s41537-020-00110-7. NPJ Schizophr. 2020. PMID: 32848142 Free PMC article. - Diuretic, Natriuretic, and Vasodepressor Activity of a Lipid Fraction Enhanced in Medium of Cultured Mouse Medullary Interstitial Cells by a Selective Fatty Acid Amide Hydrolase Inhibitor.
Daneva Z, Dempsey SK, Ahmad A, Li N, Li PL, Ritter JK. Daneva Z, et al. J Pharmacol Exp Ther. 2019 Feb;368(2):187-198. doi: 10.1124/jpet.118.252320. Epub 2018 Dec 7. J Pharmacol Exp Ther. 2019. PMID: 30530623 Free PMC article. - Circulating Endocannabinoids and Mortality in Hemodialysis Patients.
Moradi H, Park C, Streja E, Argueta DA, DiPatrizio NV, You AS, Rhee CM, Vaziri ND, Kalantar-Zadeh K, Piomelli D. Moradi H, et al. Am J Nephrol. 2020;51(2):86-95. doi: 10.1159/000505444. Epub 2020 Jan 14. Am J Nephrol. 2020. PMID: 31935741 Free PMC article. - Overactivation of the Endocannabinoid System in Adolescence Disrupts Adult Adipose Organ Function in Mice.
Jung KM, Lin L, Piomelli D. Jung KM, et al. Cells. 2024 Mar 6;13(5):461. doi: 10.3390/cells13050461. Cells. 2024. PMID: 38474425 Free PMC article. - Cannabinoids and the kidney: effects in health and disease.
Park F, Potukuchi PK, Moradi H, Kovesdy CP. Park F, et al. Am J Physiol Renal Physiol. 2017 Nov 1;313(5):F1124-F1132. doi: 10.1152/ajprenal.00290.2017. Epub 2017 Jul 26. Am J Physiol Renal Physiol. 2017. PMID: 28747360 Free PMC article. Review.
References
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. - PubMed
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. - PubMed
- Di Marzo V, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. - PubMed