Tertiary lymphoid organs in infection and autoimmunity - PubMed (original) (raw)
Review
Tertiary lymphoid organs in infection and autoimmunity
Katrijn Neyt et al. Trends Immunol. 2012 Jun.
Abstract
The lymph nodes (LNs) and spleen have an optimal structure that allows the interaction between T cells, B cells and antigen-presenting dendritic cells (DCs) on a matrix made up by stromal cells. Such a highly organized structure can also be formed in tertiary lymphoid organs (TLOs) at sites of infection or chronic immune stimulation. This review focuses on the molecular mechanisms of TLO formation and maintenance, the controversies surrounding the nature of the inducing events, and the functions of these structures in infection, transplantation and autoimmunity.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
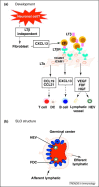
Development and structure of secondary lymphoid structures. (a) During SLO development, the earliest instructive signal is from a neuronal cell that induces the local fibroblasts to upregulate CXCL13 and thus attract LTi cells. These cells express LTβ and instruct the local fibroblasts to become LTo cells that start producing chemokines for B cells (CXCL13), T cells and DCs (CCL19 and CCL21). At the same time, the fibroblasts upregulate cell adhesion molecules to allow LTis and recruited T and B cells to stick together. Local angiogenic growth factors are also made to allow the development of high endothelial venules (HEV), as well as afferent and efferent lymph vessels. (b) As a result, a well-organized structure with a separate B cell (blue) and T cell zone (red) is formed. This allows cell–cell contact at the appropriate time; and entry and exit of lymphocytes and antigen via the HEVs and lymphatics.
Figure 2
Development and structure of tertiary lymphoid structures. (a) During chronic immune responses or transplant rejection, DCs continuously present antigens to T and B cells. Activated B cells express LTβ and can act as potent LTi-like cells, to induce an LTo phenotype in local myofibroblasts. Alternatively, chronic antigen presentation by DCs might also lead to induction of a Th17 cell response that can also induce TLOs through unclear mechanisms. Th17 cells are held in place via interactions with podoplanin. (b) As in SLOs, TLOs are divided into discrete B and T cell areas. Although, fully formed TLO structures often contain only a single T cell area and a larger B cell area, in which GC reactions can be seen. These also contain DCs and FDCs. At the periphery, an elaborate network of lymphatics (Lyve1+ and Prox1+) is commonly found but it is currently unknown if these are afferent or efferent lymphatics.
Similar articles
- High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation.
Ruddle NH. Ruddle NH. Front Immunol. 2016 Nov 9;7:491. doi: 10.3389/fimmu.2016.00491. eCollection 2016. Front Immunol. 2016. PMID: 27881983 Free PMC article. Review. - Basics of Inducible Lymphoid Organs.
Ruddle NH. Ruddle NH. Curr Top Microbiol Immunol. 2020;426:1-19. doi: 10.1007/82_2020_218. Curr Top Microbiol Immunol. 2020. PMID: 32588229 Review. - Tertiary Lymphoid Structures Among the World of Noncanonical Ectopic Lymphoid Organizations.
Silva-Sanchez A, Randall TD, Meza-Perez S. Silva-Sanchez A, et al. Methods Mol Biol. 2018;1845:1-15. doi: 10.1007/978-1-4939-8709-2_1. Methods Mol Biol. 2018. PMID: 30141004 Review. - The role of dendritic cells in tertiary lymphoid structures: implications in cancer and autoimmune diseases.
Reste M, Ajazi K, Sayi-Yazgan A, Jankovic R, Bufan B, Brandau S, Bækkevold ES, Petitprez F, Lindstedt M, Adema GJ, Almeida CR. Reste M, et al. Front Immunol. 2024 Oct 11;15:1439413. doi: 10.3389/fimmu.2024.1439413. eCollection 2024. Front Immunol. 2024. PMID: 39483484 Free PMC article. Review. - Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma.
Stranford S, Ruddle NH. Stranford S, et al. Front Immunol. 2012 Nov 30;3:350. doi: 10.3389/fimmu.2012.00350. eCollection 2012. Front Immunol. 2012. PMID: 23230435 Free PMC article.
Cited by
- Maturation of Tertiary Lymphoid Structures.
Shu DH, Sidiropoulos DN. Shu DH, et al. Methods Mol Biol. 2025;2864:43-55. doi: 10.1007/978-1-0716-4184-2_3. Methods Mol Biol. 2025. PMID: 39527216 Review. - Tertiary Lymphoid Structures in Central Nervous System Disorders.
Vaccaro A, de Alves Pereira B, van de Walle T, Dimberg A. Vaccaro A, et al. Methods Mol Biol. 2025;2864:21-42. doi: 10.1007/978-1-0716-4184-2_2. Methods Mol Biol. 2025. PMID: 39527215 Review. - Inflammation, tertiary lymphoid structures, and lung cancer: a bibliometric analysis.
Liu X, Liu H, Huang L, Lin L, Cai Q, Xiang Y, Liu Z, Zeng S, He J, Liang W. Liu X, et al. Transl Lung Cancer Res. 2024 Oct 31;13(10):2636-2648. doi: 10.21037/tlcr-24-350. Epub 2024 Oct 28. Transl Lung Cancer Res. 2024. PMID: 39507023 Free PMC article. - Tertiary lymphoid structure-related score as a predictor for survival prognosis and immunotherapy response in head and neck squamous cell carcinoma.
Wu F, Cao H, Ren S, Wu J, Liu X, Li Q, Xu Q, Chen J, Wang R, Chen S, Kuang S, Xia B, Li Y, Wang L, Li J, Li B, Li J, Lan T. Wu F, et al. Front Immunol. 2024 Oct 18;15:1483497. doi: 10.3389/fimmu.2024.1483497. eCollection 2024. Front Immunol. 2024. PMID: 39493749 Free PMC article. - Tertiary Lymphoid Structures in Microorganism-Related Cancer.
Deng S, Yang X, He L, Hou Y, Meng H. Deng S, et al. Cancers (Basel). 2024 Oct 12;16(20):3464. doi: 10.3390/cancers16203464. Cancers (Basel). 2024. PMID: 39456558 Free PMC article. Review.
References
- Turley S.J. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 2010;10:813–825. - PubMed
- van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. - PubMed
- Roozendaal R., Mebius R.E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 2011;29:23–43. - PubMed
- Eberl G. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources