Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain - PubMed (original) (raw)
Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain
João M Bráz et al. Neuron. 2012.
Abstract
Neuropathic pain is a chronic debilitating disease characterized by mechanical allodynia and spontaneous pain. Because symptoms are often unresponsive to conventional methods of pain treatment, new therapeutic approaches are essential. Here, we describe a strategy that not only ameliorates symptoms of neuropathic pain but is also potentially disease modifying. We show that transplantation of immature telencephalic GABAergic interneurons from the mouse medial ganglionic eminence (MGE) into the adult mouse spinal cord completely reverses the mechanical hypersensitivity produced by peripheral nerve injury. Underlying this improvement is a remarkable integration of the MGE transplants into the host spinal cord circuitry, in which the transplanted cells make functional connections with both primary afferent and spinal cord neurons. By contrast, MGE transplants were not effective against inflammatory pain. Our findings suggest that MGE-derived GABAergic interneurons overcome the spinal cord hyperexcitability that is a hallmark of nerve injury-induced neuropathic pain.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
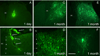
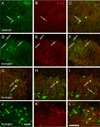
Figure 1. MGE-derived transplants are viable in the spinal cord of naïve, adult mice
MGE-derived cells constitutively express GFP (green). (A–B) One day after transplantation, the transplant consisted of an aggregate of GFP+ MGE cells, at or near the injection site. Occasionally we observed isolated cells that migrated from the injection site as well as cells at the surface of the cord (arrows in B). The transplants were injected unilaterally and we never observed GFP+ cells that migrated to the other side of the cord. (C–E) The majority of GFP+ MGE cells were located in deep laminae (III–V). (F) MGE cells extended long processes throughout the spinal cord. Scale bar equals 200 µm in A and C, 50 µm in B, D and E and 25 µm in F.
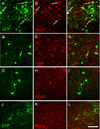
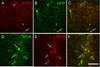
Figure 2. MGE-derived cells differentiate into neurons
(A–C) One month after transplantation, most GFP+ (green) MGE cells expressed NeuN (red), a marker of neurons. (D–F) GFP+ (green) MGE cells did not express Iba1 (red), a marker of microglia, or GFAP (G–I; red), a marker of astrocytes. (J–K) In contrast, one week after transplantation, MGE cells did not co-stain for NeuN (red). Arrows point to examples of NeuN-GFP+, double-labeled cells. Scale bar equals 50 µm.
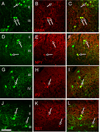
Figure 3. MGE-derived neurons assume a GABAergic phenotype
MGE-derived cells (green) co-expressed markers (red) of cortical GABAergic interneurons, including GABA (A–C), neuropeptide Y (NPY, D–F), parvalbumin (PV, G–I) and somatostatin (SST, J–L). Arrows point to examples of double-labeled cells. Scale bar equals 50 µm.
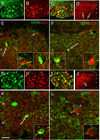
Figure 4. Transplanted MGE-derived neurons receive inputs from both myelinated and unmyelinated primary afferent neurons
(A–F) We transplanted MGE cells into the spinal cord of mice that express the inducible transneuronal tracer WGA (red) in a mixed, unmyelinated and myelinated, NF200+ (green) population of DRG neurons (A,C). In these mice WGA is released throughout the dorsal horn of the spinal cord (laminae I–V), where it was taken up by local neurons (arrows in D), including the MGE-derived cells (green, E–F). Insets show high magnifications of neurons from E and F. (G–L) When we transplanted MGE cells into the spinal cord of ZWX mice that expressed the WGA selectively in myelinated DRG neurons (NF200+, G–I), we also detected transneuronal transfer of WGA (arrows in J), including the MGE-derived cells (green, K). Here, most WGA labeling was concentrated in deeper laminae (III–V), where cutaneous myelinated primary afferents terminate (compare WGA-labeling patterns in D and J). We also observed WGA+, but GFP− neurons that were enveloped by terminals that derive from the MGE cells (L). Insets show high magnifications of neurons from K and L. Scale bars equals 200 µm in A–C and G–I; 100 µm in D and J; 30 µm in E, F, K and L.
Figure 5. Transplanted MGE-derived neurons are activated by innocuous and noxious peripheral stimulation
(A–C) Walking for 90’ on a rotarod induced expression of Fos (red) in MGE cells, indicating that innocuous peripheral stimuli (in this case conveyed by myelinated primary afferents) activate the MGE transplants. (D–L) Hindpaw injection of formalin (1%) also induces expression of Fos (red) in a large number of spinal cord neurons, including GFP+ (green) MGE-derived neurons, 1 month after transplantation (D–I) but not at 1 week post-transplantation (J–L). Arrows point to examples of Fos and GFP+ double-labeled cells. Scale bars equals 50 µm in A–C and G–L, 100 µm in D–F.
Figure 6. Transplanted MGE-derived neurons establish connections with host spinal cord neurons
(A–C) We infected MGE cells with a lentivirus that expresses the tracer WGA and the reporter mCherry and transplanted them in naïve, adult mice. Two weeks after transplantation, ~30% of GFP+ (green, I) MGE cells expressed WGA (red). Importantly, we detected WGA+/GFP− (arrowheads in A–C) but also WGA+/mCh− (arrows in D–F) neurons in the spinal cord of transplanted mice indicating that spinal cord neurons had taken up the tracer after its release from MGE cells (see also Figure S3). Arrowheads in D–F point to examples of WGA+/mCh+ double labeled cells. Scale bars equals 100 µm in A–C and 50 µm in D–F.
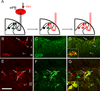
Figure 7. Transplanted MGE-derived neurons integrate into spinal cord circuits that engage lamina I projection neurons
(A) Injection of the retrograde transneuronal viral tracer pseudorabies (PRV, red) in the external lateral parabrachial nucleus (elPB; left panel) results in infection of spinal cord projection neurons that target the elPB (projection neurons “become” red; middle panel). Subsequent retrograde transfer of PRV labels spinal cord neurons that are upstream of the infected projection neurons (spinal neurons, including GFP+ (green) MGE cell “become” red; right panel). (B–D) PRV+ (red) projection neurons of spinal cord lamina I (arrows) and PRV+ interneurons of laminae II–III (arrowheads). Note the extensive arborization of GFP-positive terminals around retrogradely PRV-labeled neurons (D, inset; see also Supplementary Figure 5). (E–G) MGE transplanted cells (green) are among the cells that are retrogradely infected by PRV (red), indicating that MGE cells have integrated into a neuronal circuit that influences projection neurons of lamina I. Arrowheads in E point to retrogradely infected PRV+/GFP− spinal cord interneurons. Arrows point to examples of PRV+/GFP+ double-labeled cells. Arrowheads Scale bars equals 100 µm in B–D and 50 µm in E–G.
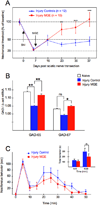
Figure 8. MGE-derived neurons reverse the mechanical allodynia produced by partial nerve injury but do not affect inflammatory pain
(A) Changes of mechanical threshold (allodynia; von Frey test) after partial sciatic nerve injury (SNI) and after MGE transplantation are reported as percent of pre-SNI baseline ± SEM. One day after SNI, there is a dramatic reduction of the mechanical threshold of the hindpaw ipsilateral to the injury. In SNI animals that only received cell medium, the marked mechanical allodynia persisted throughout the 4 week observation period (blue line). In contrast, there was a gradual reduction of the mechanical allodynia in MGE-transplanted animals (red line), with a complete reversal at one month (day 37 post SNI). The difference between mechanical thresholds of the 2 groups was statistically significant 2 weeks after transplantation. (B) Compared to naïve animals, we found that after SNI, there is a small but significant decrease in the spinal cord levels of GAD65, but not of GAD67 mRNA, ipsilateral to the nerve injury. In the MGE transplanted group, both GAD65 and GAD67 levels were significantly greater than those in medium-injected nerve-injured mice. However, the levels of GAD mRNA in the MGE transplanted and naïve mice did not differ. Asterisks (*) indicate statistically significant differences between groups, with * = p < 0.05 and ** = p < 0.01. (C) Transplanting MGE cells did not reduce pain behaviors in the formalin test. Although there was a slight decrease throughout the second phase of testing, the effect was not statistically significant.
Comment in
- Pain: Transplanted precursors halt neuropathic pain.
Whalley K. Whalley K. Nat Rev Neurosci. 2012 Jun 13;13(7):447. doi: 10.1038/nrn3283. Nat Rev Neurosci. 2012. PMID: 22691911 No abstract available.
Similar articles
- Functional Synaptic Integration of Forebrain GABAergic Precursors into the Adult Spinal Cord.
Etlin A, Bráz JM, Kuhn JA, Wang X, Hamel KA, Llewellyn-Smith IJ, Basbaum AI. Etlin A, et al. J Neurosci. 2016 Nov 16;36(46):11634-11645. doi: 10.1523/JNEUROSCI.2301-16.2016. J Neurosci. 2016. PMID: 27852772 Free PMC article. - Long-term, dynamic synaptic reorganization after GABAergic precursor cell transplantation into adult mouse spinal cord.
Llewellyn-Smith IJ, Basbaum AI, Bráz JM. Llewellyn-Smith IJ, et al. J Comp Neurol. 2018 Feb 15;526(3):480-495. doi: 10.1002/cne.24346. Epub 2017 Nov 13. J Comp Neurol. 2018. PMID: 29134656 Free PMC article. - TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord.
Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB. Kim YH, et al. Neuron. 2012 May 24;74(4):640-7. doi: 10.1016/j.neuron.2012.02.039. Neuron. 2012. PMID: 22632722 - Impaired Autophagy of GABAergic Interneurons in Neuropathic Pain.
Yin Y, Yi MH, Kim DW. Yin Y, et al. Pain Res Manag. 2018 Sep 25;2018:9185368. doi: 10.1155/2018/9185368. eCollection 2018. Pain Res Manag. 2018. PMID: 30356379 Free PMC article. Review. - Neuropathic Pain: Sensory Nerve Injury or Motor Nerve Injury?
Liu XG, Pang RP, Zhou LJ, Wei XH, Zang Y. Liu XG, et al. Adv Exp Med Biol. 2016;904:59-75. doi: 10.1007/978-94-017-7537-3_5. Adv Exp Med Biol. 2016. PMID: 26900063 Review.
Cited by
- Synaptic integration of transplanted interneuron progenitor cells into native cortical networks.
Howard MA, Baraban SC. Howard MA, et al. J Neurophysiol. 2016 Aug 1;116(2):472-8. doi: 10.1152/jn.00321.2016. Epub 2016 May 25. J Neurophysiol. 2016. PMID: 27226453 Free PMC article. - The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain.
Li C, Lei Y, Tian Y, Xu S, Shen X, Wu H, Bao S, Wang F. Li C, et al. Mol Pain. 2019 Jan-Dec;15:1744806919847366. doi: 10.1177/1744806919847366. Mol Pain. 2019. PMID: 30977423 Free PMC article. Review. - Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury.
Lorenzo LE, Magnussen C, Bailey AL, St Louis M, De Koninck Y, Ribeiro-da-Silva A. Lorenzo LE, et al. Mol Pain. 2014 Sep 4;10:57. doi: 10.1186/1744-8069-10-57. Mol Pain. 2014. PMID: 25189404 Free PMC article. - Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation.
Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JL, Alvarez-Buylla A, Huang Y. Tong LM, et al. J Neurosci. 2014 Jul 16;34(29):9506-15. doi: 10.1523/JNEUROSCI.0693-14.2014. J Neurosci. 2014. PMID: 25031394 Free PMC article. - Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity.
Bráz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI. Bráz JM, et al. Pain. 2015 Jun;156(6):1084-1091. doi: 10.1097/j.pain.0000000000000152. Pain. 2015. PMID: 25760475 Free PMC article.
References
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical