Monoaminergic modulation of photoreception in ascidian: evidence for a proto-hypothalamo-retinal territory - PubMed (original) (raw)
Monoaminergic modulation of photoreception in ascidian: evidence for a proto-hypothalamo-retinal territory
Florian Razy-Krajka et al. BMC Biol. 2012.
Abstract
Background: The retina of craniates/vertebrates has been proposed to derive from a photoreceptor prosencephalic territory in ancestral chordates, but the evolutionary origin of the different cell types making the retina is disputed. Except for photoreceptors, the existence of homologs of retinal cells remains uncertain outside vertebrates.
Methods: The expression of genes expressed in the sensory vesicle of the ascidian Ciona intestinalis including those encoding components of the monoaminergic neurotransmission systems, was analyzed by in situ hybridization or in vivo transfection of the corresponding regulatory elements driving fluorescent reporters. Modulation of photic responses by monoamines was studied by electrophysiology combined with pharmacological treatments.
Results: We show that many molecular characteristics of dopamine-synthesizing cells located in the vicinity of photoreceptors in the sensory vesicle of the ascidian Ciona intestinalis are similar to those of amacrine dopamine cells of the vertebrate retina. The ascidian dopamine cells share with vertebrate amacrine cells the expression of the key-transcription factor Ptf1a, as well as that of dopamine-synthesizing enzymes. Surprisingly, the ascidian dopamine cells accumulate serotonin via a functional serotonin transporter, as some amacrine cells also do. Moreover, dopamine cells located in the vicinity of the photoreceptors modulate the light-off induced swimming behavior of ascidian larvae by acting on alpha2-like receptors, instead of dopamine receptors, supporting a role in the modulation of the photic response. These cells are located in a territory of the ascidian sensory vesicle expressing genes found both in the retina and the hypothalamus of vertebrates (six3/6, Rx, meis, pax6, visual cycle proteins).
Conclusion: We propose that the dopamine cells of the ascidian larva derive from an ancestral multifunctional cell population located in the periventricular, photoreceptive field of the anterior neural tube of chordates, which also gives rise to both anterior hypothalamus and the retina in craniates/vertebrates. It also shows that the existence of multiple cell types associated with photic responses predates the formation of the vertebrate retina.
Figures
Figure 1
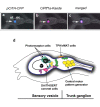
Expression of CiPtf1a in dopamine cells of the sensory vesicle of C. intestinalis larva. Dorsal views of heads of C. intestinalis larvæ expressing CFP in TH cells (pSP-Ci-TH-CFP) (a), as well as the Kaede reporter under the control of the CiPtf1a promoter (pSP-Ci-Ptf1a Kaede) (b) in the sensory vesicle of ascidian larva; merged image (c). Ot : otolith and Oc : ocellus. (anterior to the left, scale bar, 50 µm). Ot, otolith and Oc, ocellus. (scale bar, 50 μm). (c) Schematic drawing of a lateral view of the head and neck of an ascidian larva (the tail is not shown). The head CNS is shaded and divided into sensory vesicle the trunk ganglion. The neuronal populations expressing identified neurotransmitters are grossly shown. The DA coronet cells (pink), also expressing SERT and Ptf1a, are in close relationships with the population III of photoreceptors and one population of cholinergic neurons (yellow). They send processes toward glutamatergic (purple) and GABAergic (green) neurons. The TPH/vMAT expressing cells, which are localized dorso-laterally to the CNS are shown only on the right side. The central motor pattern generator and mostly the cholinergic output neurons are shown.
Figure 2
Expression of monoaminergic markers in C. intestinalis embryos. Lateral (a, c, e, g, i, k, anterior to the right) and dorsal views (b, d, f, h, j, l, anterior to the right) of late-tail-bud-stage embryos. (a, b) β-galactosidase activity driven by the CiTH cis-regulatory sequence (pCiTH-LacZ) (dopamine cells) is detected in the ventral sensory vesicle. (c-l) In situ hybridization of the indicated probes. White arrows point to the pigmented otolith. (c, d) CiGch is expressed in two domains, one corresponding to the CiTH expressing cell population within the sensory vesicle and the other to the CiTph territory flanking the trunk ganglion (small black arrows). CiAadc-a (e, f) and CiSERT (g, h) are expressed in the CiTH domain, while CivMat-a (i, j) is expressed at the same location as CiTph, outside of the nervous system (small black arrows). To accurately localize these transcripts, we used CiNut (k, l) as a pan-neural marker expressed in the CNS including the sensory vesicle (SV) and the trunk ganglion (TG). Sense probes gave no signal (data not shown). Scale bar = 20 μm.
Figure 3
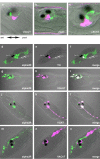
Promoter-driven co-expression of TH, AADC and SERT in the same cells of sensory vesicle. Views of heads of C. intestinalis larvae co-expressing CFP in TH cells (pSP-Ci-TH-CFP, a, d, g) and Kaede under the control of the AADC promoter (pSP-Ci-AADC-Kaede, b) GCH promoter (pSP-Ci-GCH-Kaede, e) or the SERT promoter (pSP-Ci-SERT-Kaede, h). Merged images (c, f, i) demonstrate the co-localization of TH, AADC, GCH and _SERT_-driven fluorescent reporters in the DA cells of the Ciona sensory vesicle. Ot, otolith and Oc, ocellus. Ant., anterior to the left, Post, posterior to the right for all the images.
Figure 4
Expression of CiTH and of Ci-ADRα2a receptor in the neuronal network of C. intestinalis larva. Confocal sections of larvae co-electroporated with pCi-TH-WGA and constructs reporting expression of the following neurotransmitter markers: (a, d) glutamate transporter (pCi-vGluT-eGFP), (b, e) GABA transporter (pCi-vGaT-eGFP) and (c, f) acetylcholine transporter (pCi-VAchT-eGFP). Localizations of WGA (red) and eGFP (green) were visualized by immunofluorescent staining with an anti-WGA antibody and an anti-GFP antibody, respectively. Merged images (a, b, c) corresponds to merged images with Nomarski channel (d, e, f). A, anterior, P, posterior. (Scale bar, 25 μm in (a, b), 10 μm in c.). (c-n) Confocal sections of larvae with co-electroporated pCi-ADRα2a-Venus and constructs reporting expression of the following markers of neurotransmitter systems: (c, d, e) dopamine (pCiTH-Kaede), (f, g, h) glutamate (pCi-vGluT-Kaede), (i, j, k) GABA (pCi-vGaT-Kaede) and (l, m, n) acetylcholine (pCi-VAchT-Kaede). Localization of Venus (green) (c, f, i, l) Kaede (red) (d, g, j, m) were respectively visualized by immunofluorescent staining with an anti-Kaede antibody and an anti-GFP antibody. (d, h, k, n) merged images of anti-Kaede and anti-Venus staining. These are representative example of all the performed experiments (n = 5 to 15) depending on the couple of transgenes used.
Figure 5
Effect of α2 ADR agonist and antagonist on light-induced swimming response in C. intestinalis larva. Muscle field potentials are recorded from the larval tail (see Methods) as an index of its contractile states. Response of Ciona larva to a five-second exposure to dark in control (upper trace), drug-treated (middle trace), and washed (lower trace) preparations are shown for fluoxetine (20 μM, left panel), dexmetedomidine (middle panel) and atipamizole (right panel). The blocking effect on light-off induced swimming of fluoxetine, is reproduced by the ADRα2 antagonist, whereas the ADRα2 agonist had an opposite effect, suggesting these receptors are mediating the effect of released monoamines (probably DA) on the photomotor response. These are representative example of all the experiments we have performed (n = 5 to 10 depending on the drug used).
Figure 6
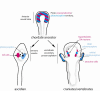
Schematic representation of the proposed evolutionary scenario for the emergence of the retina and hypothalamus in craniates/vertebrates. The anterior neural tube of a chordate ancestor contained a periventricular photoreceptor cells (ciliated cells, at least in part glutamatergic) intermingled with neuroendocrine cells synthesizing dopamine and neuropeptides. These cells could also be connected to the CNS. These cell types are lining the anterior neural ventricle and contact the CSF. A reminiscent but derived situation is found in modern protochordates such as ascidian (inferior left part of the drawing) where photoreceptor cells line the ventricle and are adjacent to the DA cells of the sensory vesicle, which make coronets inside the ventricle. DA cells are able to modulate the motor response to light. The situation could be very similar in the amphioxus neural tube, based on current description of the photoreceptor and DA cells. In craniates/vertebrates, the optic vesicle becomes separated from the anterior hypothalamus at the end of the neurulation process and bulged out of the neural tube to reach the lateral neuroectodermal epithelium and the lens placode, leading to new morphogenetic movements and to the inversion of the retina. The retina comprised several cell types inherited from the protochordate ancestor, including at least photoreceptor cells, pigmented epithelium and amacrine DA cells.
Similar articles
- The dopamine-synthesizing cells in the swimming larva of the tunicate Ciona intestinalis are located only in the hypothalamus-related domain of the sensory vesicle.
Moret F, Christiaen L, Deyts C, Blin M, Joly JS, Vernier P. Moret F, et al. Eur J Neurosci. 2005 Jun;21(11):3043-55. doi: 10.1111/j.1460-9568.2005.04147.x. Eur J Neurosci. 2005. PMID: 15978015 - Spatio-temporal regulation of Rx and mitotic patterns shape the eye-cup of the photoreceptor cells in Ciona.
Oonuma K, Kusakabe TG. Oonuma K, et al. Dev Biol. 2019 Jan 15;445(2):245-255. doi: 10.1016/j.ydbio.2018.11.011. Epub 2018 Nov 28. Dev Biol. 2019. PMID: 30502325 - Revised lineage of larval photoreceptor cells in Ciona reveals archetypal collaboration between neural tube and neural crest in sensory organ formation.
Oonuma K, Tanaka M, Nishitsuji K, Kato Y, Shimai K, Kusakabe TG. Oonuma K, et al. Dev Biol. 2016 Dec 1;420(1):178-185. doi: 10.1016/j.ydbio.2016.10.014. Epub 2016 Oct 24. Dev Biol. 2016. PMID: 27789227 - Photoreceptive systems in ascidians.
Kusakabe T, Tsuda M. Kusakabe T, et al. Photochem Photobiol. 2007 Mar-Apr;83(2):248-52. doi: 10.1562/2006-07-11-IR-965. Photochem Photobiol. 2007. PMID: 16939365 Review. - Origin of the vertebrate visual cycle.
Takimoto N, Kusakabe T, Tsuda M. Takimoto N, et al. Photochem Photobiol. 2007 Mar-Apr;83(2):242-7. doi: 10.1562/2006-06-30-IR-957. Photochem Photobiol. 2007. PMID: 16930093 Review.
Cited by
- Photoreceptor specialization and the visuomotor repertoire of the primitive chordate Ciona.
Salas P, Vinaithirthan V, Newman-Smith E, Kourakis MJ, Smith WC. Salas P, et al. J Exp Biol. 2018 Apr 11;221(Pt 7):jeb177972. doi: 10.1242/jeb.177972. J Exp Biol. 2018. PMID: 29511068 Free PMC article. - Single-cell transcriptome profiling of the Ciona larval brain.
Sharma S, Wang W, Stolfi A. Sharma S, et al. Dev Biol. 2019 Apr 15;448(2):226-236. doi: 10.1016/j.ydbio.2018.09.023. Epub 2018 Oct 28. Dev Biol. 2019. PMID: 30392840 Free PMC article. - Transcription Factors of the bHLH Family Delineate Vertebrate Landmarks in the Nervous System of a Simple Chordate.
Negrón-Piñeiro LJ, Wu Y, Di Gregorio A. Negrón-Piñeiro LJ, et al. Genes (Basel). 2020 Oct 26;11(11):1262. doi: 10.3390/genes11111262. Genes (Basel). 2020. PMID: 33114624 Free PMC article. Review. - Comparative single-cell transcriptomic analysis reveals putative differentiation drivers and potential origin of vertebrate retina.
Zeng X, Gyoja F, Cui Y, Loza M, Kusakabe TG, Nakai K. Zeng X, et al. NAR Genom Bioinform. 2024 Nov 12;6(4):lqae149. doi: 10.1093/nargab/lqae149. eCollection 2024 Sep. NAR Genom Bioinform. 2024. PMID: 39534499 Free PMC article. - Cellular identity and Ca2+ signaling activity of the non-reproductive GnRH system in the Ciona intestinalis type A (Ciona robusta) larva.
Okawa N, Shimai K, Ohnishi K, Ohkura M, Nakai J, Horie T, Kuhara A, Kusakabe TG. Okawa N, et al. Sci Rep. 2020 Oct 29;10(1):18590. doi: 10.1038/s41598-020-75344-7. Sci Rep. 2020. PMID: 33122709 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials