Chemoselective ligation of sulfinic acids with aryl-nitroso compounds - PubMed (original) (raw)
Chemoselective ligation of sulfinic acids with aryl-nitroso compounds
Mauro Lo Conte et al. Angew Chem Int Ed Engl. 2012.
Abstract
Making a comeback: The inefficient condensation of sulfinic acid and aryl nitroso compounds has been transformed into a chemoselective process that converts sulfinic acid into stable cyclic sulfonamide analogues (see scheme). This ligation proceeds rapidly under aqueous conditions in high yield, and lays the groundwork for the development of sulfinic acid detection methods in biological systems.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Figure 1
Resonance and structures of sulfinate anions in this study. Boc = _tert_-butoxycarbonyl.
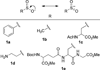
Scheme 1
Condensation reaction between a C-nitroso compound and aryl sulfinic acid.
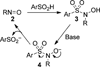
Scheme 2
Proposed mechanism of N-sulfonylbenzisoxazolone formation.
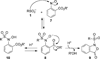
Scheme 3
Control experiments for the ligation of sulfinic acid by 7f in the presence of excess thiols.
Similar articles
- A Chemical Approach for the Detection of Protein Sulfinylation.
Lo Conte M, Lin J, Wilson MA, Carroll KS. Lo Conte M, et al. ACS Chem Biol. 2015 Aug 21;10(8):1825-30. doi: 10.1021/acschembio.5b00124. Epub 2015 Jun 17. ACS Chem Biol. 2015. PMID: 26039147 Free PMC article. - Harnessing Redox Cross-Reactivity To Profile Distinct Cysteine Modifications.
Majmudar JD, Konopko AM, Labby KJ, Tom CT, Crellin JE, Prakash A, Martin BR. Majmudar JD, et al. J Am Chem Soc. 2016 Feb 17;138(6):1852-9. doi: 10.1021/jacs.5b06806. Epub 2016 Feb 5. J Am Chem Soc. 2016. PMID: 26780921 Free PMC article. - Palladium-catalyzed desulfitative Heck-type reaction of aryl sulfinic acids with alkenes.
Wang GW, Miao T. Wang GW, et al. Chemistry. 2011 May 16;17(21):5787-90. doi: 10.1002/chem.201100539. Epub 2011 Apr 13. Chemistry. 2011. PMID: 21491525 No abstract available. - Sulfinate derivatives: dual and versatile partners in organic synthesis.
Aziz J, Messaoudi S, Alami M, Hamze A. Aziz J, et al. Org Biomol Chem. 2014 Dec 28;12(48):9743-59. doi: 10.1039/c4ob01727g. Org Biomol Chem. 2014. PMID: 25354469 Review. - The sulfinic acid switch in proteins.
Jacob C, Holme AL, Fry FH. Jacob C, et al. Org Biomol Chem. 2004 Jul 21;2(14):1953-6. doi: 10.1039/b406180b. Epub 2004 Jun 29. Org Biomol Chem. 2004. PMID: 15254616 Review.
Cited by
- Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins.
Brewer TF, Garcia FJ, Onak CS, Carroll KS, Chang CJ. Brewer TF, et al. Annu Rev Biochem. 2015;84:765-90. doi: 10.1146/annurev-biochem-060614-034018. Annu Rev Biochem. 2015. PMID: 26034893 Free PMC article. Review. - A Chemical Approach for the Detection of Protein Sulfinylation.
Lo Conte M, Lin J, Wilson MA, Carroll KS. Lo Conte M, et al. ACS Chem Biol. 2015 Aug 21;10(8):1825-30. doi: 10.1021/acschembio.5b00124. Epub 2015 Jun 17. ACS Chem Biol. 2015. PMID: 26039147 Free PMC article. - Nitro-sulfinate Reductive Coupling to Access (Hetero)aryl Sulfonamides.
Gatarz SE, Griffiths OM, Esteves HA, Jiao W, Morse P, Fisher EL, Blakemore DC, Ley SV. Gatarz SE, et al. J Org Chem. 2024 Feb 2;89(3):1898-1909. doi: 10.1021/acs.joc.3c02557. Epub 2024 Jan 18. J Org Chem. 2024. PMID: 38239107 Free PMC article. - Redox Signaling by Reactive Electrophiles and Oxidants.
Parvez S, Long MJC, Poganik JR, Aye Y. Parvez S, et al. Chem Rev. 2018 Sep 26;118(18):8798-8888. doi: 10.1021/acs.chemrev.7b00698. Epub 2018 Aug 27. Chem Rev. 2018. PMID: 30148624 Free PMC article. Review. - Functional group divergence and the structural basis of acridine photocatalysis revealed by direct decarboxysulfonylation.
Nguyen VT, Haug GC, Nguyen VD, Vuong NTH, Karki GB, Arman HD, Larionov OV. Nguyen VT, et al. Chem Sci. 2022 Mar 21;13(14):4170-4179. doi: 10.1039/d2sc00789d. eCollection 2022 Apr 6. Chem Sci. 2022. PMID: 35440976 Free PMC article.
References
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th ed. New York: Oxford University Press; 2007.
- Reddie KG, Carroll KS. Curr. Opin. Chem. Biol. 2008;12:746–754. - PubMed
- Jacob C, Giles GI, Giles NM, Sies H. Angew. Chem. 2003;115:4890–4907. Angew. Chem. Int. Ed.2003, 42, 4742 – 4758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources