Autophagy regulates Wolbachia populations across diverse symbiotic associations - PubMed (original) (raw)
Autophagy regulates Wolbachia populations across diverse symbiotic associations
Denis Voronin et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Wolbachia are widespread and abundant intracellular symbionts of arthropods and filarial nematodes. Their symbiotic relationships encompass obligate mutualism, commensalism, parasitism, and pathogenicity. A consequence of these diverse associations is that Wolbachia encounter a wide range of host cells and intracellular immune defense mechanisms of invertebrates, which they must evade to maintain their populations and spread to new hosts. Here we show that autophagy, a conserved intracellular defense mechanism and regulator of cell homeostasis, is a major immune recognition and regulatory process that determines the size of Wolbachia populations. The regulation of Wolbachia populations by autophagy occurs across all distinct symbiotic relationships and can be manipulated either chemically or genetically to modulate the Wolbachia population load. The recognition and activation of host autophagy is particularly apparent in rapidly replicating strains of Wolbachia found in somatic tissues of Drosophila and filarial nematodes. In filarial nematodes, which host a mutualistic association with Wolbachia, the use of antibiotics such as doxycycline to eliminate Wolbachia has emerged as a promising approach to their treatment and control. Here we show that the activation of host nematode autophagy reduces bacterial loads to the same magnitude as antibiotic therapy; thus we identify a bactericidal mode of action targeting Wolbachia that can be exploited for the development of chemotherapeutic agents against onchocerciasis, lymphatic filariasis, and heartworm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
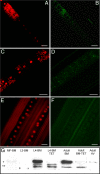
Fig. 1.
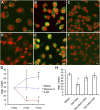
Association of ATG8a expression and Wolbachia in the filarial nematode B. malayi. (A_–_F) ATG8a (green in B, D, and F) colocalized with Wolbachia clusters (small red spots in A and C; large red structures are nematode nuclei) throughout the lateral chord cytoplasm of adult female B. malayi (A_–_D) and is absent from naturally _Wolbachia_-free A. viteae (E and F). (Scale bars: 50 μm. (G) Western blot (composite image) of the ATG8a protein in B. malayi (BM) and A. viteae (AV). MF-BM, B. malayi microfilaria; L3-BM, B. malayi L3; L4-BM, B. malayi L4; L4-BM-TET, B. malayi L4 treated with tetracycline in vivo for 14 d; Adult-BM, protein extract from untreated adult females; Adult-BM-TET protein extract from adult females treated with tetracycline in vivo for 6 wk. *ATG8a cytosolic form; **cleaved membrane-associated forms.
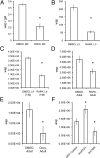
Fig. 2.
qPCR analysis of Wolbachia numbers in B. malayi after in vitro treatment with rapamycin (A_–_D), doxycycline (E), or siRNA (F). (A) Ratio of wsp/gst in microfilaria after 5 d of treatment with rapamycin (RAPA). (B) Number of wsp copies in L3 larvae treated for 5 d. (C) Number of wsp copies in L4 larvae treated for 5 d. (D) Number of wsp copies per worm in adult females treated with rapamycin or DMSO (control) for 7 d. (E) Number of wsp copies per worm in adult females treated with doxycycline (Doxy) and DMSO (control). (F) Number of wsp copies per worm in adult females treated with siRNA (bmTOR or bmATG1) or GFP as a control. *P < 0.001.
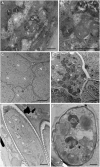
Fig. 3.
Morphological effects on B. malayi treated with rapamycin. Micrographs of hypodermal chord cells (A and B), developing embryos (C and D), and stretched microfilaria (E and F) in the uterus of adult females treated with rapamycin and control. A, B, D, and F show rapamycin-treated samples; C and E show control samples. The arrow in B indicates the fusion of the lysosome and bacteria. B, bacteria; Bi, degenerated bacteria; L, lysosomes; N, nuclei. (Scale bars: 1 μm in A and B; 15 μm in C_–_F.)
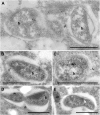
Fig. 4.
Ultrastructural localization of ATG8a protein in B. malayi and Wolbachia on the bacterial vacuole (A_–_E), bacterial cell wall (B and D), and in the bacterial cell matrix (A, B, C, E). b, bacteria; m, mitochondria. (Scale bars: 1 μm.)
Fig. 5.
Regulation of autophagy controls ATG8a expression and Wolbachia load in A. albopictus C6/36 cells. (A_–_F) Detection of ATG8a (green) in C6/36 (wAlbB) cells (A_–_C) and uninfected C6/36 cells (D_–_F) during the treatment. (A and D) Control (DMSO-treated) cells. (B and E) Rapamycin-induced cells display up-regulated ATG8a signals. (C and F) 3-MA–treated cells show suppressed expression of ATG8a. (Scale bars: 5 μm). (G and H) qPCR analysis of Wolbachia (WSP) and host actin gene copies in mosquito cells after treatment. (G) Ratio of wsp:actin in the C6/36 (wAlbB) cells treated with rapamycin, 3-MA, or DMSO (control). (H) Ratio of wsp:actin in the C6/36 (wAlbB) cells exposed to starvation (Star-Med) and treated with autophagy inhibitors Wortmannin (WM) or 3-MA. *P < 0.001.
Fig. 6.
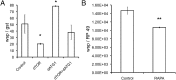
Autophagy activation controls Wolbachia populations in Drosophila. (A) Effects of siRNA treatment on _w_MelPop populations in Drosophila cells (PC15). Ratio of wsp:RP49 in PC15 (_w_MelPop) cells. (B) Reduction of the wsp:RP49 ratio in D. melanogaster (w1118) naturally infected with _w_MelPop and treated with rapamycin (RAPA).
Fig. 7.
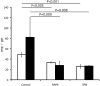
qPCR analysis of Wolbachia number in B. malayi after in vivo treatment with rapamycin (RAPA) or spermidine (SPN) and in controls. Reductions of Wolbachia were seen in worms treated with inducers of autophagy. White bars indicate wsp:gst ratio in L4 larvae (treated for 14 d), black bars indicate wsp:gst ratio in adult females treated for 35 d.
Fig. P1.
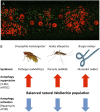
Autophagy regulates Wolbachia populations. (A) Autophagy membrane protein ATG8a (green) is associated with Wolbachia (small red dots) in the hypodermal chord cells of the filarial nematode B. malayi. (Large red structures are nematode nuclei,) (B) Natural populations of Wolbachia in diverse symbioses and hosts are regulated by autophagy. Chemical (3-methyladenine, 3-MA) or genetic (siRNA atg1) suppression of autophagy increases Wolbachia loads, whereas activation of autophagy by rapamycin or siRNA tor decreases bacterial load in host cells.
Similar articles
- Glucose and Glycogen Metabolism in Brugia malayi Is Associated with Wolbachia Symbiont Fitness.
Voronin D, Bachu S, Shlossman M, Unnasch TR, Ghedin E, Lustigman S. Voronin D, et al. PLoS One. 2016 Apr 14;11(4):e0153812. doi: 10.1371/journal.pone.0153812. eCollection 2016. PLoS One. 2016. PMID: 27078260 Free PMC article. - Pyruvate produced by Brugia spp. via glycolysis is essential for maintaining the mutualistic association between the parasite and its endosymbiont, Wolbachia.
Voronin D, Schnall E, Grote A, Jawahar S, Ali W, Unnasch TR, Ghedin E, Lustigman S. Voronin D, et al. PLoS Pathog. 2019 Sep 30;15(9):e1008085. doi: 10.1371/journal.ppat.1008085. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31568486 Free PMC article. - Wolbachia bacterial endosymbionts of filarial nematodes.
Taylor MJ, Bandi C, Hoerauf A. Taylor MJ, et al. Adv Parasitol. 2005;60:245-84. doi: 10.1016/S0065-308X(05)60004-8. Adv Parasitol. 2005. PMID: 16230105 Review. - Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes.
Landmann F, Voronin D, Sullivan W, Taylor MJ. Landmann F, et al. PLoS Pathog. 2011 Nov;7(11):e1002351. doi: 10.1371/journal.ppat.1002351. Epub 2011 Nov 3. PLoS Pathog. 2011. PMID: 22072969 Free PMC article. - The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis.
Bouchery T, Lefoulon E, Karadjian G, Nieguitsila A, Martin C. Bouchery T, et al. Clin Microbiol Infect. 2013 Feb;19(2):131-40. doi: 10.1111/1469-0691.12069. Clin Microbiol Infect. 2013. PMID: 23398406 Review.
Cited by
- New insights into the transovarial transmission of the symbiont Rickettsia in whiteflies.
Shan H, Liu Y, Luan J, Liu S. Shan H, et al. Sci China Life Sci. 2021 Jul;64(7):1174-1186. doi: 10.1007/s11427-020-1801-7. Epub 2020 Sep 30. Sci China Life Sci. 2021. PMID: 33021711 - High pressure freezing/freeze substitution fixation improves the ultrastructural assessment of Wolbachia endosymbiont-filarial nematode host interaction.
Fischer K, Beatty WL, Weil GJ, Fischer PU. Fischer K, et al. PLoS One. 2014 Jan 17;9(1):e86383. doi: 10.1371/journal.pone.0086383. eCollection 2014. PLoS One. 2014. PMID: 24466066 Free PMC article. - Programmed cell death pathways as targets for developing antifilarial drugs: Lessons from the recent findings.
Das NC, Chakraborty P, Nandy S, Dey A, Malik T, Mukherjee S. Das NC, et al. J Cell Mol Med. 2023 Oct;27(19):2819-2840. doi: 10.1111/jcmm.17913. Epub 2023 Aug 22. J Cell Mol Med. 2023. PMID: 37605891 Free PMC article. Review. - Jamestown Canyon virus is transmissible by Aedes aegypti and is only moderately blocked by Wolbachia co-infection.
Lau MJ, Dutra HLC, Jones MJ, McNulty BP, Diaz AM, Ware-Gilmore F, McGraw EA. Lau MJ, et al. PLoS Negl Trop Dis. 2023 Sep 5;17(9):e0011616. doi: 10.1371/journal.pntd.0011616. eCollection 2023 Sep. PLoS Negl Trop Dis. 2023. PMID: 37669272 Free PMC article. - Iron necessity: the secret of Wolbachia's success?
Gill AC, Darby AC, Makepeace BL. Gill AC, et al. PLoS Negl Trop Dis. 2014 Oct 16;8(10):e3224. doi: 10.1371/journal.pntd.0003224. eCollection 2014 Oct. PLoS Negl Trop Dis. 2014. PMID: 25329055 Free PMC article. Review.
References
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol. 2005;60:245–284. - PubMed
- Dedeine F, Boulétreau M, Vavre F. Wolbachia requirement for oogenesis: Occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida. Heredity (Edinb) 2005;95:394–400. - PubMed
- Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. - PubMed
- McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources