Multimodal photoacoustic ophthalmoscopy in mouse - PubMed (original) (raw)
Multimodal photoacoustic ophthalmoscopy in mouse
Wei Song et al. J Biophotonics. 2013 Jun.
Abstract
Photoacoustic ophthalmoscopy (PAOM) is a novel imaging technology that measures optical absorption in the retina. The capability of PAOM can be further enhanced if it could image mouse eyes, because mouse models are widely used for various retinal diseases. The challenges in achieving high-quality imaging of mouse retina, however, come from the much smaller eyeball size. Here, we report an optimized imaging system, which integrates PAOM, spectral-domain optical coherence tomography (SD-OCT), and autofluorescence-scanning laser ophthalmoscopy (AF-SLO), for mouse eyes. Its multimodal capability was demonstrated by imaging transgenic Nrl-GFP mice that express green fluorescent protein (GFP) in photoreceptors. SD-OCT provided guidance of optical alignment for PAOM and AF-SLO, and complementary contrast with high depth-resolution retinal cross sections. PAOM visualized the retinal vasculature and retinal pigment epithelium melanin, and AF-SLO measured GFP-expressing in retinal photoreceptors. The in vivo imaging results were verified by histology and confocal microscopy.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
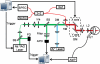
Figure 1
Schematic diagram of the multimodal retinal imaging system. SLED: superluminescent laser diode; Ref: OCT reference arm; 2×2: 50:50 optical fiber coupler; SPEC: spectrometer; BS: beam splitter; DM: dichroic mirror; HM; hot mirror; GM: 2D galvanometer mirrors; AMP: amplifier; UT: ultrasonic transducer; PD: photodiode; APD: avalanche photodetector.
Figure 2
In vivo multimodal retinal imaging. (a) and (d) are en face SD-OCT images; (b) and (e) are PAOM fundus images; (c) and (f) are AF-SLO fundus images. The images on the left are acquired from a transgenic Nrl-GFP mouse and the images on the right are acquired from a control mouse. The arrows in Figure 2b point out some smaller vessel shadows that were visible in PAOM but invisible in SD-OCT and AF-SLO. Bar: 300 μm.
Figure 3
Comparison of (a) SD-OCT cross-section, (b) PAOM B-scan, and (c) 1D AF-SLO profile acquired from the same position. RNFL/GC: retinal nerve fiber layer and ganglion cell layer; IP: inner plexiform layer; IN: inner nuclear layer; OP: outer plexiform layer; ON: outer nuclear layer; ELM: external limiting membrane; IS/OS: the inner and outer segment of the photoreceptors; RPE/C: the complex of retinal pigment epithelium and choroid. The arrows pointing out the four retinal vessels show good registration among the three imaging modalities. Bar: 100 μm.
Figure 4
Retinal histological of the (a) GFP-expressing eye and the (b) control eye. The anatomic retinal layers were resolved by DAPI staining (blue) in both eyes. The turquoise band locates in ONL represents GFP expression. GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer. The locations pointed out by arrows indicate no GFP expression. Bar: 50 μm.
Figure 5
Comparison between ex vivo flat-mount confocal microscopy results and the in vivo results. (a) Confocal microscopy of the whole-mount retina stained by Brn-3b. The trace of major retinal vessels is labeled by numbers. (b) Confocal microscopy of GFP expression in the whole-mount retina. The photoreceptors with GFP expression appeared as bright turquoise regions. (c) Overlaid in vivo PAOM and AF-SLO images within the region highlighted by the dashed-box in Figure. 2b. The retinal vessels in PAOM are pseudo-colored in red and the AF-SLO image is pseudo-colored in green. Representative photoreceptors in absence of GFP were highlighted by arrows. Bar: 100 μm.
Similar articles
- Integrated photoacoustic ophthalmoscopy and spectral-domain optical coherence tomography.
Song W, Wei Q, Jiao S, Zhang HF. Song W, et al. J Vis Exp. 2013 Jan 15;(71):e4390. doi: 10.3791/4390. J Vis Exp. 2013. PMID: 23354081 Free PMC article. - Integrating photoacoustic ophthalmoscopy with scanning laser ophthalmoscopy, optical coherence tomography, and fluorescein angiography for a multimodal retinal imaging platform.
Song W, Wei Q, Liu T, Kuai D, Burke JM, Jiao S, Zhang HF. Song W, et al. J Biomed Opt. 2012 Jun;17(6):061206. doi: 10.1117/1.JBO.17.6.061206. J Biomed Opt. 2012. PMID: 22734736 Free PMC article. - Fundus camera guided photoacoustic ophthalmoscopy.
Liu T, Li H, Song W, Jiao S, Zhang HF. Liu T, et al. Curr Eye Res. 2013 Dec;38(12):1229-34. doi: 10.3109/02713683.2013.815219. Epub 2013 Jul 25. Curr Eye Res. 2013. PMID: 24131226 Free PMC article. - Photoacoustic ophthalmoscopy for in vivo retinal imaging: current status and prospects.
Zhang HF, Puliafito CA, Jiao S. Zhang HF, et al. Ophthalmic Surg Lasers Imaging. 2011 Jul;42 Suppl(0):S106-15. doi: 10.3928/15428877-20110627-10. Ophthalmic Surg Lasers Imaging. 2011. PMID: 21790106 Free PMC article. Review. - The fundus photo has met its match: optical coherence tomography and adaptive optics ophthalmoscopy are here to stay.
Morgan JI. Morgan JI. Ophthalmic Physiol Opt. 2016 May;36(3):218-39. doi: 10.1111/opo.12289. Ophthalmic Physiol Opt. 2016. PMID: 27112222 Free PMC article. Review.
Cited by
- Advanced nanomaterials for imaging of eye diseases.
Nguyen VP, Hu J, Zhe J, Ramasamy S, Ahmed U, Paulus YM. Nguyen VP, et al. ADMET DMPK. 2024 Feb 20;12(2):269-298. doi: 10.5599/admet.2182. eCollection 2024. ADMET DMPK. 2024. PMID: 38720929 Free PMC article. Review. - Retinal safety evaluation of photoacoustic microscopy.
Li Y, Zhang W, Nguyen VP, Khan NW, Xia X, Wang X, Paulus YM. Li Y, et al. Exp Eye Res. 2021 Jan;202:108368. doi: 10.1016/j.exer.2020.108368. Epub 2020 Nov 24. Exp Eye Res. 2021. PMID: 33242491 Free PMC article. - High Resolution Multimodal Photoacoustic Microscopy and Optical Coherence Tomography Visualization of Choroidal Vascular Occlusion.
Nguyen VP, Li Y, Henry J, Zhang W, Wang X, Paulus YM. Nguyen VP, et al. Int J Mol Sci. 2020 Sep 5;21(18):6508. doi: 10.3390/ijms21186508. Int J Mol Sci. 2020. PMID: 32899568 Free PMC article. - Advances in Retinal Optical Imaging.
Li Y, Xia X, Paulus YM. Li Y, et al. Photonics. 2018 Jun;5(2):9. doi: 10.3390/photonics5020009. Epub 2018 Apr 27. Photonics. 2018. PMID: 31321222 Free PMC article. - Imaging retinal melanin: a review of current technologies.
Lapierre-Landry M, Carroll J, Skala MC. Lapierre-Landry M, et al. J Biol Eng. 2018 Dec 4;12:29. doi: 10.1186/s13036-018-0124-5. eCollection 2018. J Biol Eng. 2018. PMID: 30534199 Free PMC article. Review.
References
- Pang IH, Clark AF. Animal Models for Retinal Diseases. Humana press; New York: 2010. p. 25.
- Chang B, Hawes NL, Hurd RE, Wang J, Howell D, Davisson MT, Roderick TH, Nusinowitz S, Heckenlively JR. Vis. Neurosci. 2005;22:587–593. - PubMed
Publication types
MeSH terms
Grants and funding
- R01EY019325/EY/NEI NIH HHS/United States
- RC4EY021357/EY/NEI NIH HHS/United States
- R01EY019951/EY/NEI NIH HHS/United States
- R01EY019034/EY/NEI NIH HHS/United States
- R01 EY019325/EY/NEI NIH HHS/United States
- R01 EY019034/EY/NEI NIH HHS/United States
- RC4 EY021357/EY/NEI NIH HHS/United States
- R01 EY019951/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources