Neurosteroids and GABA-A Receptor Function - PubMed (original) (raw)
Neurosteroids and GABA-A Receptor Function
Mingde Wang. Front Endocrinol (Lausanne). 2011.
Abstract
Neurosteroids represent a class of endogenous steroids that are synthesized in the brain, the adrenals, and the gonads and have potent and selective effects on the GABAA-receptor. 3α-hydroxy A-ring reduced metabolites of progesterone, deoxycorticosterone, and testosterone are positive modulators of GABA(A)-receptor in a non-genomic manner. Allopregnanolone (3α-OH-5α-pregnan-20-one), 5α-androstane-3α, 17α-diol (Adiol), and 3α5α-tetrahydrodeoxycorticosterone (3α5α-THDOC) enhance the GABA-mediated Cl(-) currents acting on a site (or sites) distinct from the GABA, benzodiazepine, barbiturate, and picrotoxin binding sites. 3α5α-P and 3α5α-THDOC potentiate synaptic GABA(A)-receptor function and activate δ-subunit containing extrasynaptic receptors that mediate tonic currents. On the contrary, 3β-OH pregnane steroids and pregnenolone sulfate (PS) are GABA(A)-receptor antagonists and induce activation-dependent inhibition of the receptor. The activities of neurosteroid are dependent on brain regions and types of neurons. In addition to the slow genomic action of the parent steroids, the non-genomic, and rapid actions of neurosteroids play a significant role in the GABA(A)-receptor function and shift in mood and memory function. This review describes molecular mechanisms underlying neurosteroid action on the GABA(A)-receptor, mood changes, and cognitive functions.
Keywords: GABAA-receptor; THDOC; allopregnanolone; cognition; mood; pregnenolone sulfate; premenstrual dysphoric disorder.
Figures
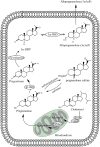
Figure 1
Biosynthesis of allopregnanolone and pregnenolone sulfate (PS) from cholesterol within the neuron or glial cell. Enzymes involved are P450 side-chain cleavage (P450scc), 3α-hydroxysteroid dehydrogenase (3α-HSD), 3β-hydroxysteroid dehydrogenase (3β-HSD) and 5α-reductase. Transport of cholesterol across the mitochondrial membrane is enhanced by the steroidogenic acute-regulatory (StAR) protein and the mitochondrial benzodiazepine receptor (MBR). 5α-DHP represents 5α-dihydroprogesterone.
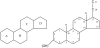
Figure 2
Features of general structure of the neurosteroid. Figure shows intact four ring system that often contains hydroxyl side chains as sterols. Hydroxyl groups are denoted α if they are oriented above the plane (solid line), and α if they are oriented below the plane (dashed line). Stereoisomerism and structural modifications were discussed at carbon 3, 5, 6, 7, 17, 20, and 21 positions.
Similar articles
- Neurosteroid modulation of recombinant rat alpha5beta2gamma2L and alpha1beta2gamma2L GABA(A) receptors in Xenopus oocyte.
Rahman M, Lindblad C, Johansson IM, Bäckström T, Wang MD. Rahman M, et al. Eur J Pharmacol. 2006 Oct 10;547(1-3):37-44. doi: 10.1016/j.ejphar.2006.07.039. Epub 2006 Jul 27. Eur J Pharmacol. 2006. PMID: 16934248 - The effects of neurosteroids on rat behavior and 3H-muscimol binding in the brain.
Członkowska AI, Sienkiewicz-Jarosz H, Siemiatkowski M, Bidziński A, Płaźnik A. Członkowska AI, et al. Pharmacol Biochem Behav. 1999 Aug;63(4):639-46. doi: 10.1016/s0091-3057(99)00030-1. Pharmacol Biochem Behav. 1999. PMID: 10462193 - Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy.
Reddy DS. Reddy DS. Front Endocrinol (Lausanne). 2011 Oct 5;2:38. doi: 10.3389/fendo.2011.00038. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654805 Free PMC article. - GABAA receptor modulating steroid antagonists (GAMSA) are functional in vivo.
Johansson M, Strömberg J, Ragagnin G, Doverskog M, Bäckström T. Johansson M, et al. J Steroid Biochem Mol Biol. 2016 Jun;160:98-105. doi: 10.1016/j.jsbmb.2015.10.019. Epub 2015 Oct 30. J Steroid Biochem Mol Biol. 2016. PMID: 26523675 Review. - Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs.
Barbaccia ML. Barbaccia ML. Crit Rev Neurobiol. 2004;16(1-2):67-74. doi: 10.1615/critrevneurobiol.v16.i12.70. Crit Rev Neurobiol. 2004. PMID: 15581401 Review.
Cited by
- Elevated Neurosteroids in the Lateral Thalamus Relieve Neuropathic Pain in Rats with Spared Nerve Injury.
Zhang M, Liu J, Zhou MM, Wu H, Hou Y, Li YF, Yin Y, Zheng L, Liu FY, Yi M, Wan Y. Zhang M, et al. Neurosci Bull. 2016 Aug;32(4):311-22. doi: 10.1007/s12264-016-0044-7. Epub 2016 Jun 21. Neurosci Bull. 2016. PMID: 27325509 Free PMC article. - Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism.
Kazdoba TM, Hagerman RJ, Zolkowska D, Rogawski MA, Crawley JN. Kazdoba TM, et al. Psychopharmacology (Berl). 2016 Jan;233(2):309-23. doi: 10.1007/s00213-015-4115-7. Epub 2015 Nov 3. Psychopharmacology (Berl). 2016. PMID: 26525567 Free PMC article. - Steroid precursors, steroids, neuroactive steroids, and neurosteroids concentrations in serum and saliva of healthy neonatal heifer Holstein calves.
Aleman M, Chigerwe M, Varga A, Madigan JE. Aleman M, et al. J Vet Intern Med. 2020 Nov;34(6):2767-2775. doi: 10.1111/jvim.15957. Epub 2020 Nov 17. J Vet Intern Med. 2020. PMID: 33201530 Free PMC article. - Direct Structural Insights into GABAA Receptor Pharmacology.
Kim JJ, Hibbs RE. Kim JJ, et al. Trends Biochem Sci. 2021 Jun;46(6):502-517. doi: 10.1016/j.tibs.2021.01.011. Epub 2021 Mar 3. Trends Biochem Sci. 2021. PMID: 33674151 Free PMC article. Review. - Plasma concentrations of steroid precursors, steroids, neuroactive steroids, and neurosteroids in healthy neonatal foals from birth to 7 days of age.
Aleman M, McCue PM, Chigerwe M, Madigan JE. Aleman M, et al. J Vet Intern Med. 2019 Sep;33(5):2286-2293. doi: 10.1111/jvim.15618. Epub 2019 Sep 5. J Vet Intern Med. 2019. PMID: 31489708 Free PMC article.
References
- Adkins C. E., Pillai G. V., Kerby J., Bonnert T. P., Haldon C., McKernan R. M., Gonzalez J. E., Oades K., Whiting P. J., Simpson P. B. (2001). alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 276, 38934–3893910.1074/jbc.M104318200 - DOI - PubMed
- Amin J., Weiss D. S. (1994). Homomeric rho 1 GABA channels: activation properties and domains. Recept. Channels 2, 227–236 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources