Emerging role of protein-protein transnitrosylation in cell signaling pathways - PubMed (original) (raw)
Review
Emerging role of protein-protein transnitrosylation in cell signaling pathways
Tomohiro Nakamura et al. Antioxid Redox Signal. 2013.
Abstract
Significance: Protein S-nitrosylation, a covalent reaction of a nitric oxide (NO) group with a critical protein thiol (or more properly thiolate anion), mediates an important form of redox-related signaling as well as aberrant signaling in disease states.
Recent advances: A growing literature suggests that over 3000 proteins are S-nitrosylated in cell systems. Our laboratory and several others have demonstrated that protein S-nitrosylation can regulate protein function by directly inhibiting catalytically active cysteines, by reacting with allosteric sites, or via influencing protein-protein interaction. For example, S-nitrosylation of critical cysteine thiols in protein-disulfide isomerase and in parkin alters their activity, thus contributing to protein misfolding in Parkinson's disease.
Critical issues: However, the mechanism by which specific protein S-nitrosylation occurs in cell signaling pathways is less well investigated. Interestingly, the recent discovery of protein-protein transnitrosylation reactions (transfer of an NO group from one protein to another) has revealed a unique mechanism whereby NO can S-nitrosylate a particular set of protein thiols, and represents a major class of nitrosylating/denitrosylating enzymes in mammalian systems. In this review, we will discuss recent evidence for transnitrosylation reactions between (i) hemoglobin/anion exchanger 1, (ii) thioredoxin/caspase-3, (iii) X-linked inhibitor of apoptosis/caspase-3, (iv) GAPDH-HDAC2/SIRT1/DNA-PK, and (v) Cdk5/dynamin related protein 1 (Drp1). This review also discusses experimental techniques useful in characterizing protein-protein transnitrosylations.
Future directions: Elucidation of additional transnitrosylation cascades will further our understanding of the enzymes that catalyze nitrosation, thereby contributing to NO-mediated signaling pathways.
Figures
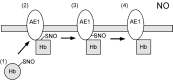
FIG. 1.
The pathway exporting NO from erythrocytes requires transnitrosylation of AE1 by SNO-Hb. Hb, which is _S_-nitrosylated at Cys93 (forming SNO-Hb), undergoes conformational change from the R-state to T-state (1), allowing binding to the cytoplasmic tail of AE1 (2). SNO-Hb transnitrosylates AE1, forming SNO-AE1 (3). SNO-AE1 then facilitates export of the NO group out of red blood cells, enabling NO to act as a vasorelaxing factor (4). AE1, anion exchanger 1; Hb, hemoglobin; NO, nitric oxide; SNO, _S_-nitrosothiol.
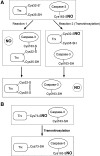
FIG. 2.
Trx mediates denitrosylation and transnitrosylation of caspase-3. (A) Possible denitrosylation schemes for _S_-nitrosylated caspase-3 by Trx. In reaction 1, Cys32 of Trx attacks SNO-caspase-3, leading to the formation of an intermolecular disulfide between caspase-3 and Trx. This reaction allows denitrosylation of caspase-3. Subsequently, an intramolecular disulfide bridge forms between Cys32 and Cys35 in Trx, releasing the reduced form of caspase-3. In reaction 2, SNO-caspase-3 acts as a nitrosylase, transnitrosylating Trx. This reaction also precipitates denitrosylation of caspase-3. (B) Transfer of an NO group from Trx to caspase-3. After Trx is _S_-nitrosylated at Cys73 (forming SNO-Trx-Cys73), SNO-Trx-Cys73 transnitrosylates Cys163 of caspase-3. This reaction results in the inhibition of caspase-3. Trx, thioredoxin.
FIG. 3.
Schematic illustration of the mechanism of SNO-XIAP–mediated apoptotic cell death. (1) Under normal conditions (non-nitrosative conditions), XIAP efficiently binds to and inhibits caspase activity. Additionally, XIAP serves as an E3 ligase that ubiquitinates caspases and thus targets caspases for proteasomal degradation. (2) Under conditions of nitrosative stress, NO inactivates the E3 ligase activity of XIAP _via S_-nitrosylation, thus stabilizing caspases, and sensitizing neurons to apoptotic stimuli, which is mediated by Smac. (3) Caspases are constitutively _S_-nitrosylated, serving as a transnitrosylase for XIAP, producing SNO-XIAP in cells undergoing apoptotic cell death. XIAP, X-linked inhibitor of apoptosis.
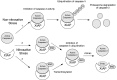
FIG. 4.
Signaling pathways mediated by S -nitrosylated GAPDH. Toxic levels of NO can _S_-nitrosylate GAPDH in the cytosol. _S_-Nitrosylation of GAPDH promotes interaction between GAPDH and Siah, facilitating nuclear translocation of the GAPDH-Siah1 complex. NO preferentially _S_-nitrosylates GOSPEL to form a SNO-GOSPEL/GAPDH complex when NO levels are relatively low, preventing GAPDH from interacting with Siah1. In the nucleus, the GAPDH-Siah1 complex contributes to neuronal cell injury and death via promoting degradation of nuclear proteins, such as N-CoR, and stimulating expression of genes downstream to p300/CBP, such as p53. Moreover, SNO-GAPDH serves as a nuclear nitrosylase, producing SNO-SIRT1, SNO-HDAC2, and SNO-DNA-PK.
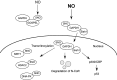
FIG. 5.
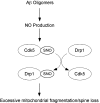
Schema of SNO-Cdk5 contributing to transnitrosylation of Drp1 and the pathogenesis of AD. In AD, oligomeric Aβ can promote NO production, which results in the formation of SNO-Cdk5. _S_-Nitrosylation of Cdk5 per se activates its activity, thus contributing to neuronal injury. In addition, SNO-Cdk5 transfers an NO group to mitochondrial fission protein Drp1. _S_-Nitrosylated Drp1 manifests excessive fission activity, causing abnormal mitochondrial fragmentation and compromise in mitochondrial bioenergetics, thus impairing synaptic form and function. Aβ, amyloid-β; AD, Alzheimer's disease; Drp1, dynamin related protein 1.
FIG. 6.
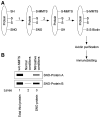
Biotin-switch technique for the calculation of the relative redox potential and the associated change in Gibbs free energy. (A) Overview of the biotin-switch assay. The biotin-switch assay consists of three steps: (1) blocking of free thiol groups by MMTS, (2) conversion of SNOs to free thiols by ascorbate, and (3) labeling newly formed free thiols (formerly _S_-nitrosylated) by biotin-HPDP. Biotinylated proteins are purified by avidin-agarose, and proteins are eluted and analyzed by SDS-PAGE/immunoblotting. (B) Schematic gel image for calculation of standard redox potentials and Gibbs free energy. Cell or tissue lysates are subjected to the biotin-switch assay. MMTS is used to block free thiols during the assay for _S_-nitrosylated protein. “Total thiol protein” represents the relative amount of total protein obtained by the biotin-switch assay performed in the absence of MMTS. “SNO-protein” represents the relative amount of _S_-nitrosylated protein obtained by the biotin-switch assay. Band intensities in lanes 1 and 3 are measured for calculating the standard redox potential using a variant of the Nernst equation and the Gibbs free energy (see text for further details). MMTS, _S_-methyl methanethiosulfonate.
Similar articles
- Protein Transnitrosylation Signaling Networks Contribute to Inflammaging and Neurodegenerative Disorders.
Nakamura T, Oh CK, Zhang X, Tannenbaum SR, Lipton SA. Nakamura T, et al. Antioxid Redox Signal. 2021 Sep 1;35(7):531-550. doi: 10.1089/ars.2021.0081. Epub 2021 Jun 21. Antioxid Redox Signal. 2021. PMID: 33957758 Free PMC article. Review. - The role of thioredoxin in the regulation of cellular processes by S-nitrosylation.
Sengupta R, Holmgren A. Sengupta R, et al. Biochim Biophys Acta. 2012 Jun;1820(6):689-700. doi: 10.1016/j.bbagen.2011.08.012. Epub 2011 Aug 22. Biochim Biophys Acta. 2012. PMID: 21878369 Review. - Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation.
Sengupta R, Holmgren A. Sengupta R, et al. Antioxid Redox Signal. 2013 Jan 20;18(3):259-69. doi: 10.1089/ars.2012.4716. Epub 2012 Aug 10. Antioxid Redox Signal. 2013. PMID: 22702224 Review. - Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling.
Stomberski CT, Hess DT, Stamler JS. Stomberski CT, et al. Antioxid Redox Signal. 2019 Apr 1;30(10):1331-1351. doi: 10.1089/ars.2017.7403. Epub 2018 Jan 10. Antioxid Redox Signal. 2019. PMID: 29130312 Free PMC article. Review. - Evidence against Stable Protein S-Nitrosylation as a Widespread Mechanism of Post-translational Regulation.
Wolhuter K, Whitwell HJ, Switzer CH, Burgoyne JR, Timms JF, Eaton P. Wolhuter K, et al. Mol Cell. 2018 Feb 1;69(3):438-450.e5. doi: 10.1016/j.molcel.2017.12.019. Epub 2018 Jan 18. Mol Cell. 2018. PMID: 29358077 Free PMC article.
Cited by
- Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome.
Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M. Morris G, et al. Mol Neurobiol. 2017 Aug;54(6):4271-4291. doi: 10.1007/s12035-016-9975-2. Epub 2016 Jun 23. Mol Neurobiol. 2017. PMID: 27339878 Review. - Protein S-nitrosylation: specificity and identification strategies in plants.
Lamotte O, Bertoldo JB, Besson-Bard A, Rosnoblet C, Aimé S, Hichami S, Terenzi H, Wendehenne D. Lamotte O, et al. Front Chem. 2015 Jan 7;2:114. doi: 10.3389/fchem.2014.00114. eCollection 2014. Front Chem. 2015. PMID: 25750911 Free PMC article. Review. - Down regulation of NO signaling in Trypanosoma cruzi upon parasite-extracellular matrix interaction: changes in protein modification by nitrosylation and nitration.
Pereira M, Soares C, Canuto GA, Tavares MF, Colli W, Alves MJ. Pereira M, et al. PLoS Negl Trop Dis. 2015 Apr 9;9(4):e0003683. doi: 10.1371/journal.pntd.0003683. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25856423 Free PMC article. - Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties.
Zaffagnini M, Fermani S, Costa A, Lemaire SD, Trost P. Zaffagnini M, et al. Front Plant Sci. 2013 Nov 12;4:450. doi: 10.3389/fpls.2013.00450. eCollection 2013. Front Plant Sci. 2013. PMID: 24282406 Free PMC article. Review. - Target-selective protein S-nitrosylation by sequence motif recognition.
Jia J, Arif A, Terenzi F, Willard B, Plow EF, Hazen SL, Fox PL. Jia J, et al. Cell. 2014 Oct 23;159(3):623-34. doi: 10.1016/j.cell.2014.09.032. Epub 2014 Oct 16. Cell. 2014. PMID: 25417112 Free PMC article.
References
- Alexander C. Votruba M. Pesch UE. Thiselton DL. Mayer S. Moore A. Rodriguez M. Kellner U. Leo-Kottler B. Auburger G. Bhattacharya SS. Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. - PubMed
- Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. - PubMed
- Cartoni R. Martinou JC. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Exp Neurol. 2009;218:268–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057096/NS/NINDS NIH HHS/United States
- R01 EY05477/EY/NEI NIH HHS/United States
- R01 EY09024/EY/NEI NIH HHS/United States
- P01 ES016738/ES/NIEHS NIH HHS/United States
- P01 HD29587/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous