Fatty acids in cardiovascular health and disease: a comprehensive update - PubMed (original) (raw)
Review
Fatty acids in cardiovascular health and disease: a comprehensive update
Seth J Baum et al. J Clin Lipidol. 2012 May-Jun.
Abstract
Research dating back to the 1950s reported an association between the consumption of saturated fatty acids (SFAs) and risk of coronary heart disease. Recent epidemiological evidence, however, challenges these findings. It is well accepted that the consumption of SFAs increases low-density lipoprotein cholesterol (LDL-C), whereas carbohydrates, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) do not. High-density lipoprotein (HDL)-C increases with SFA intake. Among individuals who are insulin resistant, a low-fat, high-carbohydrate diet typically has an adverse effect on lipid profiles (in addition to decreasing HDL-C, it also increases triglyceride and LDL particle concentrations). Consequently, a moderate fat diet in which unsaturated fatty acids replace SFAs and carbohydrates are not augmented is advised to lower LDL-C; compared with a low-fat diet, a moderate-fat diet will lower triglycerides and increase HDL-C. Now, there is some new evidence that is questioning the health benefits of even MUFAs and PUFAs. In addition, in a few recent studies investigators have also failed to demonstrate expected cardiovascular benefits of marine-derived omega-3 fatty acids. To clarify the clinical pros and cons of dietary fats, the National Lipid Association held a fatty acid symposium at the 2011 National Lipid Association Scientific Sessions. During these sessions, the science regarding the effects of different fatty acid classes on coronary heart disease risk was reviewed.
Copyright © 2012 National Lipid Association. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Relationships among the PUFA. EFAs, essential fatty acids; PG, prostaglandin.
Figure 2
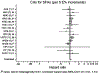
Coronary deaths in the Pooling Project of Cohort Studies on Diet and Coronary Disease. The model included intake of MUFA, PUFA, _trans_-fatty acids, carbohydrates, and protein expressed as percentages of total energy, fiber, alcohol, and cholesterol intakes; smoking; body mass index; physical activity and educational levels; history of hypertension; and ages at baseline and when questionnaire was returned. Within each study, hazard ratios with 95% CIs for the incidence of coronary events from CHD were calculated by the use of Cox proportional hazards regression with time in study as the time metric. The study-specific logs of hazard ratios were weighted by the inverse of their variances, and a combined estimate of the hazard ratios was computed by using a random effects model. The squares and horizontal lines represent the study-specific hazard ratios and 95% CIs, respectively. The area of the squares reflects the study-specific weight (inverse of the variance). The diamonds represent the combined hazard ratios and 95% CI. AHS, Adventis Health Study; ARIC, Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study; CH, carbohydrate; FMC, Finnish Mobile Clinic Health Study; GPS, Glostrup Population Study; HPFS, Health Professionals Follow-Up Study; NHSa, Nurses’ Health Study 1980; NHSb, Nurses’ Health Study 1986; VIP, Västerbotten Intervention Program; WHS, Women’s Health Study. Permission to reuse figure granted by American Society for Nutrition.
Figure 3
Meta-analysis of randomized controlled trials evaluating effects on CHD events of increasing polyunsaturated fat in place of saturated fat. CS, Coronary Survey; DART, Diet And Reinfarction Trial; LA, Los Angeles; MRC, Medical Research Council; RR, relative risk; Rx, treatment; STARS, St. Thomas’ Atherosclerosis Regression Study. Permission to reuse figure granted by PLoS Medicine.
Figure 4
Changes in blood lipid levels following replacement of carbohydrate with various fats. β reflects the change for each 1% energy isocaloric replacement. *P <.05. CHO, carbohydrate; TC, total cholesterol; TFA, _trans_-saturated fatty acids. Permission to reuse figure granted by Springer.
Figure 5
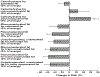
Estimated percent changes in CHD risk with isocaloric substitutions of dietary components: The Nurses’ Health Study. Permission to reuse figure granted by New England Journal of Medicine, Massachusetts Medical Society.
Figure 6
Effects on CHD risk of consuming PUFA, carbohydrate, or MUFA in place of SFA. Predicted effects were determined by changes in the TC/HDL-C ratio in short-term trials (eg, each 5% energy of PUFA replacing SFA lowers TC/HDL-C ratio by 0.16) coupled with observed associations between the TC/HDL-C ratio and CHD outcomes in middle-aged adults. Evidence for effects of dietary changes on actual CHD events came from the meta-analysis of eight randomized controlled trials for PUFA replacing SFA and from the Women’s Health Initiative randomized controlled trial for carbohydrate replacing SFA. Evidence for observed relationships of usual dietary habits with CHD events was from a pooled analysis of 11 prospective cohort studies. RCT, randomized controlled trials; RR, relative risk; TC, total cholesterol; WHI, Women’s Health Initiative. Permission to reuse figure granted by PLoS Medicine.
Figure 7
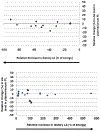
Effects of decreasing and increasing dietary LA intakes on changes in blood phospholipid AA content. Significant (P < .05) changes in AA as reported in the original papers designated as triangles; nonsignificant (_P_ > .05) AA changes as reported in the original papers designated as diamonds. PL, phospholipid. Permission to reuse figure granted by BioMed Central Ltd.
Figure 8
Forest plots of CHD death and death from all causes from a meta-analysis of randomized controlled trials in which dietary interventions increased n-6 PUFA specifically, or increased both n-3 and n-6 PUFA. (A) indicates mixed n-3/n-6 randomized controlled trial datasets providing substantial quantities of n-3 EPA + DHA and n-6 LA; (B) indicates mixed n-3/n-6 datasets providing substantial quantities of n-3 ALA and n-6 LA; (C) indicates n-6 specific datasets selectively increasing n-6 LA. Permission to reuse figure granted by Cambridge University Press.
Figure 9
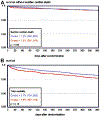
Kaplan-Meier diagrams from the OMEGA study in which 1 g/d n-3-acid ethyl esters were administered after acute MI. _P_-values are from the univariate analysis. A, Survival without sudden cardiac death during 1-year follow-up (red line, omega group; blue line, olive oil control group). B, total survival during one-year follow-up (red line, omega group; blue line, olive oil control group). Permission to reuse figure granted by the American Heart Association.
Similar articles
- Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease.
Sacks FM, Katan M. Sacks FM, et al. Am J Med. 2002 Dec 30;113 Suppl 9B:13S-24S. doi: 10.1016/s0002-9343(01)00987-1. Am J Med. 2002. PMID: 12566134 Review. - Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study.
Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Vafeiadou K, et al. Am J Clin Nutr. 2015 Jul;102(1):40-8. doi: 10.3945/ajcn.114.097089. Epub 2015 May 27. Am J Clin Nutr. 2015. PMID: 26016869 Clinical Trial. - Emerging nutrition science on fatty acids and cardiovascular disease: nutritionists' perspectives.
Kris-Etherton PM, Fleming JA. Kris-Etherton PM, et al. Adv Nutr. 2015 May 15;6(3):326S-37S. doi: 10.3945/an.114.006981. Print 2015 May. Adv Nutr. 2015. PMID: 25979506 Free PMC article. Review. - Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial.
Brassard D, Tessier-Grenier M, Allaire J, Rajendiran E, She Y, Ramprasath V, Gigleux I, Talbot D, Levy E, Tremblay A, Jones PJ, Couture P, Lamarche B. Brassard D, et al. Am J Clin Nutr. 2017 Apr;105(4):800-809. doi: 10.3945/ajcn.116.150300. Epub 2017 Mar 1. Am J Clin Nutr. 2017. PMID: 28251937 Clinical Trial. - Associations of Dietary Intake with Cardiovascular Risk in Long-Term "Plant-Based Eaters": A Secondary Analysis of a Cross-Sectional Study.
Jakše B, Godnov U, Fras Z, Fidler Mis N. Jakše B, et al. Nutrients. 2024 Mar 11;16(6):796. doi: 10.3390/nu16060796. Nutrients. 2024. PMID: 38542706 Free PMC article.
Cited by
- Chemical, Physicochemical and Sensorial Characterization of Nitrite-Free Dry-Cured Bísaro Shoulders.
Leite A, Vasconcelos L, Ferreira I, Sarmiento-García A, Domínguez R, Santos EM, Campagnol PCB, Rodrigues S, Lorenzo JM, Teixeira A. Leite A, et al. Foods. 2022 Oct 4;11(19):3079. doi: 10.3390/foods11193079. Foods. 2022. PMID: 36230155 Free PMC article. - Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases.
Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Calviello G, et al. Biomed Res Int. 2013;2013:743171. doi: 10.1155/2013/743171. Epub 2013 Apr 17. Biomed Res Int. 2013. PMID: 23691510 Free PMC article. Review. - Impact of low-trans fat compositions on the quality of conventional and fat-reduced puff pastry.
Silow C, Zannini E, Arendt EK. Silow C, et al. J Food Sci Technol. 2016 Apr;53(4):2117-26. doi: 10.1007/s13197-016-2186-z. Epub 2016 Apr 22. J Food Sci Technol. 2016. PMID: 27413242 Free PMC article. - Replacement of saturated and trans-fatty acids in the diet v. CVD risk in the light of the most recent studies.
Makarewicz-Wujec M, Dworakowska A, Kozłowska-Wojciechowska M. Makarewicz-Wujec M, et al. Public Health Nutr. 2018 Aug;21(12):2291-2300. doi: 10.1017/S1368980018000782. Epub 2018 Apr 11. Public Health Nutr. 2018. PMID: 29636118 Free PMC article. Review. - Epoxyeicosatrienoic acids and cardioprotection: the road to translation.
Oni-Orisan A, Alsaleh N, Lee CR, Seubert JM. Oni-Orisan A, et al. J Mol Cell Cardiol. 2014 Sep;74:199-208. doi: 10.1016/j.yjmcc.2014.05.016. Epub 2014 Jun 2. J Mol Cell Cardiol. 2014. PMID: 24893205 Free PMC article. Review.
References
- Mu H, Hoy CE. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105–133. - PubMed
- IUPAC-IUB Commission on Biochemical Nomenclature (CBN). The nomenclature of lipids (recommendations 1976). Eur J Biochem. 1977;79:11–21.
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–946. - PubMed
- Brenna JT, Salem N Jr., Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids (ISSFAL). Alpha-linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous