Mitochondrial distribution of neuroglobin and its response to oxygen-glucose deprivation in primary-cultured mouse cortical neurons - PubMed (original) (raw)
Mitochondrial distribution of neuroglobin and its response to oxygen-glucose deprivation in primary-cultured mouse cortical neurons
Z Yu et al. Neuroscience. 2012.
Abstract
Neuroglobin (Ngb) is a new member of the globin family and a novel endogenous neuroprotective molecule, but its neuroprotective mechanisms remain largely undefined. Previous studies suggest Ngb is both physically and functionally related to mitochondria, however without direct evidence. Our recent discovery has shown that Ngb can physically interact with a number of mitochondrial proteins. In this study we aimed to define the physical interaction between Ngb and mitochondria by determining whether there is a mitochondrial distribution of Ngb under both physiological-resting and pathological oxygen-glucose deprivation (OGD) conditions. Western blot for the first time revealed a small portion of Ngb was physically localized in mitochondria, and the relative mitochondrial Ngb level was significantly increased after OGD in primary-cultured mouse cortical neurons, indicating a translocation of Ngb into mitochondria. Complementary approaches including confocal imaging and immuno-electron microscopy confirmed Ngb distribution in mitochondria under both basal-resting condition and OGD. Inhibitors of mitochondria permeability transition pore (mPTP) and Voltage-Dependent Anion Channel (VDAC) blocked OGD-induced increase of mitochondrial Ngb level, demonstrating a possible role of mPTP in Ngb's mitochondrial translocation. We further found that Ngb overexpression-conferred neuroprotection was correlated with increased mitochondrial Ngb level, suggesting the mitochondria distribution of Ngb is clearly associated with and may contribute to Ngb's neuroprotection.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
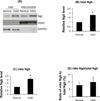
Figure 1. Quantitative analysis of Ngb distribution in mitochondria of neurons after OGD
(A) Total intracellular Ngb level and Ngb in mitochondria measured by Western blot. Mitochondria was isolated from primary cortical neurons after OGD (4 hr OGD followed by 4 hr reoxygenation) and subjected to Western blot. VDAC was used as a mitochondria protein marker, and GAPDH as a cytosol protein marker. (B) Quantitation of relative total intracellular Ngb level in OGD-treated versus non-treated neurons; (C) Quantitation of relative mitochondrial Ngb level in OGD-treated versus non-treated neurons; (D) Quantitation of the ratio of mitochondrial Ngb to total Ngb level in OGD-treated versus non-treated neurons. (n=5, *P
<c0.05)< div=""> </c0.05)<>
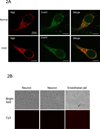
Figure 2. Ngb localization in mitochondria of neurons after OGD
(A) Double immunostaining of Ngb and Cox IV in normal or OGD-treated neurons. (Scale bar = 10 um). (B) Primary cultured mouse cortical neurons stained with secondary antibody alone (left column), with anti-Ngb antibody pre-adsorbed with recombinant Ngb protein (middle column), and endothelial cells stained with anti-Ngb antibody (right column). Cell morphology was shown on the top row and fluorescent imaging at the bottom. (C) Ngb localization in the subcellular structure of normal or OGD-treated neurons detected by immuno-electron microscopy. (Scale bar = 200 nm).
Figure 2. Ngb localization in mitochondria of neurons after OGD
(A) Double immunostaining of Ngb and Cox IV in normal or OGD-treated neurons. (Scale bar = 10 um). (B) Primary cultured mouse cortical neurons stained with secondary antibody alone (left column), with anti-Ngb antibody pre-adsorbed with recombinant Ngb protein (middle column), and endothelial cells stained with anti-Ngb antibody (right column). Cell morphology was shown on the top row and fluorescent imaging at the bottom. (C) Ngb localization in the subcellular structure of normal or OGD-treated neurons detected by immuno-electron microscopy. (Scale bar = 200 nm).
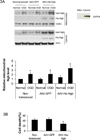
Figure 3. Ngb distribution in mitochondria of neurons with Ngb-overexpression
(A) Ngb protein level in mitochondria and cytosol from non-transduced, AAV-GFP or AAV-His-Ngb transduced neurons at normal condition or after OGD. Anti-Ngb antibody was used to measure total Ngb level in mitochondria. Anti-His tag antibody was used to measure His-Ngb level in mitochondria. The graph shows quantification of mitochondrial Ngb level (n=4, * P<0.05 versus non-transduced neurons at normal condition; # P<0.05 versus OGD-treated AAV-GFP neurons). (B) OGD-induced neuron death for non-transduced, AAV-GFP or AAV-His-Ngb transduced neurons. After 4 hr OGD plus 20 hr reoxygenation, cell death was measured by LDH release assay (n=4, * P<0.05 versus AAV-GFP transduced neurons, and non-transduced neurons).
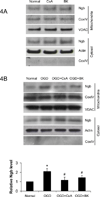
Figure 4. Effects of mPTP and VDAC inhibitors on OGD-induced Ngb translocation into mitochondria
(A) Mitochondrial Ngb levels in primary cortical neurons under basal condition and treated with mPTP inhibitors, Cyclosporine A (10 µM) and bonkrekic acid (5 µM). (B) Mitochondrial Ngb levels in primary cortical neurons after OGD and treated with mPTP inhibitors, Cyclosporine A (10 µM) and bonkrekic acid (5 µM). (C) Mitochondrial Ngb levels in primary mouse cortical neurons under basal condition and treated with VDAC inhibitors, DIDS (0.4 mM) and dextran sulfate (0.2 mM). (D) Mitochondrial Ngb levels in primary mouse cortical neurons after OGD and treated with VDAC inhibitors, DIDS (0.4 mM) and dextran sulfate (0.2 mM). (n=3, * P< 0.05 versus neurons at normal condition; # P < 0.05 versus OGD-treated neurons).
Figure 4. Effects of mPTP and VDAC inhibitors on OGD-induced Ngb translocation into mitochondria
(A) Mitochondrial Ngb levels in primary cortical neurons under basal condition and treated with mPTP inhibitors, Cyclosporine A (10 µM) and bonkrekic acid (5 µM). (B) Mitochondrial Ngb levels in primary cortical neurons after OGD and treated with mPTP inhibitors, Cyclosporine A (10 µM) and bonkrekic acid (5 µM). (C) Mitochondrial Ngb levels in primary mouse cortical neurons under basal condition and treated with VDAC inhibitors, DIDS (0.4 mM) and dextran sulfate (0.2 mM). (D) Mitochondrial Ngb levels in primary mouse cortical neurons after OGD and treated with VDAC inhibitors, DIDS (0.4 mM) and dextran sulfate (0.2 mM). (n=3, * P< 0.05 versus neurons at normal condition; # P < 0.05 versus OGD-treated neurons).
Similar articles
- Roles of Neuroglobin Binding to Mitochondrial Complex III Subunit Cytochrome c1 in Oxygen-Glucose Deprivation-Induced Neurotoxicity in Primary Neurons.
Yu Z, Zhang Y, Liu N, Yuan J, Lin L, Zhuge Q, Xiao J, Wang X. Yu Z, et al. Mol Neurobiol. 2016 Jul;53(5):3249-3257. doi: 10.1007/s12035-015-9273-4. Epub 2015 Jun 7. Mol Neurobiol. 2016. PMID: 26050086 - Neuroglobin overexpression inhibits oxygen-glucose deprivation-induced mitochondrial permeability transition pore opening in primary cultured mouse cortical neurons.
Yu Z, Liu N, Li Y, Xu J, Wang X. Yu Z, et al. Neurobiol Dis. 2013 Aug;56:95-103. doi: 10.1016/j.nbd.2013.04.015. Epub 2013 Apr 29. Neurobiol Dis. 2013. PMID: 23639789 Free PMC article. - Neuroglobin-overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation.
Yu Z, Liu J, Guo S, Xing C, Fan X, Ning M, Yuan JC, Lo EH, Wang X. Yu Z, et al. Neuroscience. 2009 Aug 18;162(2):396-403. doi: 10.1016/j.neuroscience.2009.04.055. Epub 2009 May 3. Neuroscience. 2009. PMID: 19401220 Free PMC article. - Mitochondrial mechanisms of neuroglobin's neuroprotection.
Yu Z, Poppe JL, Wang X. Yu Z, et al. Oxid Med Cell Longev. 2013;2013:756989. doi: 10.1155/2013/756989. Epub 2013 Mar 24. Oxid Med Cell Longev. 2013. PMID: 23634236 Free PMC article. Review. - Neuroglobin and neuronal cell survival.
Fiocchetti M, De Marinis E, Ascenzi P, Marino M. Fiocchetti M, et al. Biochim Biophys Acta. 2013 Sep;1834(9):1744-9. doi: 10.1016/j.bbapap.2013.01.015. Epub 2013 Jan 26. Biochim Biophys Acta. 2013. PMID: 23357651 Review.
Cited by
- Roles of Neuroglobin Binding to Mitochondrial Complex III Subunit Cytochrome c1 in Oxygen-Glucose Deprivation-Induced Neurotoxicity in Primary Neurons.
Yu Z, Zhang Y, Liu N, Yuan J, Lin L, Zhuge Q, Xiao J, Wang X. Yu Z, et al. Mol Neurobiol. 2016 Jul;53(5):3249-3257. doi: 10.1007/s12035-015-9273-4. Epub 2015 Jun 7. Mol Neurobiol. 2016. PMID: 26050086 - Mitochondrial Neuroglobin Is Necessary for Protection Induced by Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells in Astrocytic Cells Subjected to Scratch and Metabolic Injury.
Baez-Jurado E, Guio-Vega G, Hidalgo-Lanussa O, González J, Echeverria V, Ashraf GM, Sahebkar A, Barreto GE. Baez-Jurado E, et al. Mol Neurobiol. 2019 Jul;56(7):5167-5187. doi: 10.1007/s12035-018-1442-9. Epub 2018 Dec 8. Mol Neurobiol. 2019. PMID: 30536184 - Reducing lipid peroxidation attenuates stress-induced susceptibility to herpes simplex virus type 1.
Weng JY, Chen XX, Wang XH, Ye HE, Wu YP, Sun WY, Liang L, Duan WJ, Kurihara H, Huang F, Sun XX, Ou-Yang SH, He RR, Li YF. Weng JY, et al. Acta Pharmacol Sin. 2023 Sep;44(9):1856-1866. doi: 10.1038/s41401-023-01095-6. Epub 2023 May 16. Acta Pharmacol Sin. 2023. PMID: 37193755 Free PMC article. - Vulnerability of primary neurons derived from Tg2576 Alzheimer mice to oxygen and glucose deprivation: role of intraneuronal amyloid-β accumulation and astrocytes.
Baldassarro VA, Marchesini A, Giardino L, Calzà L. Baldassarro VA, et al. Dis Model Mech. 2017 May 1;10(5):671-678. doi: 10.1242/dmm.028001. Epub 2017 Feb 24. Dis Model Mech. 2017. PMID: 28237964 Free PMC article. - Carbon Monoxide-Neuroglobin Axis Targeting Metabolism Against Inflammation in BV-2 Microglial Cells.
Dias-Pedroso D, Ramalho JS, Sardão VA, Jones JG, Romão CC, Oliveira PJ, Vieira HLA. Dias-Pedroso D, et al. Mol Neurobiol. 2022 Feb;59(2):916-931. doi: 10.1007/s12035-021-02630-4. Epub 2021 Nov 19. Mol Neurobiol. 2022. PMID: 34797521
References
- Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. - PubMed
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS065998/NS/NINDS NIH HHS/United States
- R01-NS065998/NS/NINDS NIH HHS/United States
- R01-NS049476/NS/NINDS NIH HHS/United States
- R01 NS049430/NS/NINDS NIH HHS/United States
- R01 NS049476/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases