The origin and properties of extracellular DNA: from PAMP to DAMP - PubMed (original) (raw)
Review
The origin and properties of extracellular DNA: from PAMP to DAMP
David S Pisetsky. Clin Immunol. 2012 Jul.
Abstract
DNA is a polymeric macromolecule whose biological activities depend on location as well as binding to associated molecules. Inside the cell, DNA is the source of genetic information and binds histones to form nucleosomes. DNA can exit the cell, however, to enter the extracellular space primarily during cell death, either apoptosis or necrosis, as well as NETosis. While bacterial DNA is a potent immune stimulant by virtue of its CpG motifs, mammalian DNA, which is ordinarily inactive, can acquire activity by associating with nuclear, cytoplasmic and serum proteins which promote its uptake into cells to stimulate internal DNA sensors, including Toll-like receptor 9. Among these proteins, anti-DNA autoantibodies can form immune complexes with DNA to stimulate plasmacytoid dendritic cells to produce type 1 interferon. Together, these findings suggest that the immune properties of DNA are mutable and diverse, reflecting its context and the array of attached molecules.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
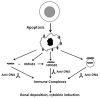
Figure 1
The generation of extracellular DNA during apoptosis. The figure illustrates the various mechanism by which DNA can leave cells during apoptosis and form immune complexes with anti-DNA antibodies. During apoptosis, the nucleus fragments and condenses and some DNA migrates to the surface to enter blebs (small dark circles on the cell surface). During late apoptosis or secondary necrosis, DNA exits the dying cell in various forms. Thus, DNA may leave cells alone or in association with HMGB1. Even if DNA and HMGB1 leave the cell separately, they may associate in the extracellular space. DNA may also leave cells in the form of microparticles (the detached form of blebs) with DNA present either on the particle surface or accessible in the particle interior to antibodies because of particle permeability. The various sources of DNA can all form immune complexes with anti-DNA autoantibodies, although the size and resulting composition of the complexes will all differ, ranging from a simple complex of DNA and anti-DNA to large, multicomponent complexes assembled on a particle structure. Some aspects of this figure, while plausible, have not yet been experimentally verified.
Similar articles
- The role of nuclear macromolecules in innate immunity.
Pisetsky DS. Pisetsky DS. Proc Am Thorac Soc. 2007 Jul;4(3):258-62. doi: 10.1513/pats.200701-027AW. Proc Am Thorac Soc. 2007. PMID: 17607009 Review. - The Alarmin Properties of DNA and DNA-associated Nuclear Proteins.
Magna M, Pisetsky DS. Magna M, et al. Clin Ther. 2016 May;38(5):1029-41. doi: 10.1016/j.clinthera.2016.02.029. Epub 2016 Mar 25. Clin Ther. 2016. PMID: 27021604 Review. - Nuclear DAMP complex-mediated RAGE-dependent macrophage cell death.
Chen R, Fu S, Fan XG, Lotze MT, Zeh HJ 3rd, Tang D, Kang R. Chen R, et al. Biochem Biophys Res Commun. 2015 Mar 13;458(3):650-655. doi: 10.1016/j.bbrc.2015.01.159. Epub 2015 Feb 13. Biochem Biophys Res Commun. 2015. PMID: 25684181 Free PMC article. - Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes.
Pisetsky D. Pisetsky D. Swiss Med Wkly. 2011 Aug 29;141:w13256. doi: 10.4414/smw.2011.13256. eCollection 2011. Swiss Med Wkly. 2011. PMID: 21877298 Free PMC article. Review. - Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE.
Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Urbonaviciute V, et al. J Exp Med. 2008 Dec 22;205(13):3007-18. doi: 10.1084/jem.20081165. Epub 2008 Dec 8. J Exp Med. 2008. PMID: 19064698 Free PMC article.
Cited by
- The crosstalk of telomere dysfunction and inflammation through cell-free TERRA containing exosomes.
Wang Z, Lieberman PM. Wang Z, et al. RNA Biol. 2016 Aug 2;13(8):690-5. doi: 10.1080/15476286.2016.1203503. Epub 2016 Jun 28. RNA Biol. 2016. PMID: 27351774 Free PMC article. Review. - Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro.
Polajzer T, Jarm T, Miklavcic D. Polajzer T, et al. Radiol Oncol. 2020 Jul 29;54(3):317-328. doi: 10.2478/raon-2020-0047. Radiol Oncol. 2020. PMID: 32726295 Free PMC article. - Oxidized cell-free DNA as a stress-signaling factor activating the chronic inflammatory process in patients with autism spectrum disorders.
Shmarina GV, Ershova ES, Simashkova NV, Nikitina SG, Chudakova JM, Veiko NN, Porokhovnik LN, Basova AY, Shaposhnikova AF, Pukhalskaya DA, Pisarev VM, Korovina NJ, Gorbachevskaya NL, Dolgikh OA, Bogush M, Kutsev SI, Kostyuk SV. Shmarina GV, et al. J Neuroinflammation. 2020 Jul 16;17(1):212. doi: 10.1186/s12974-020-01881-7. J Neuroinflammation. 2020. PMID: 32677958 Free PMC article. - Accumulation of Circulating Cell-Free CpG-Enriched Ribosomal DNA Fragments on the Background of High Endonuclease Activity of Blood Plasma in Schizophrenic Patients.
Ershova ES, Jestkova EM, Martynov AV, Shmarina GV, Umriukhin PE, Bravve LV, Zakharova NV, Kostyuk GP, Saveliev DV, Orlova MD, Bogush M, Kutsev SI, Veiko NN, Kostyuk SV. Ershova ES, et al. Int J Genomics. 2019 Aug 5;2019:8390585. doi: 10.1155/2019/8390585. eCollection 2019. Int J Genomics. 2019. PMID: 31467866 Free PMC article. - Role of platelet-derived extracellular vesicles in traumatic brain injury-induced coagulopathy and inflammation.
Liu L, Deng QJ. Liu L, et al. Neural Regen Res. 2022 Oct;17(10):2102-2107. doi: 10.4103/1673-5374.335825. Neural Regen Res. 2022. PMID: 35259815 Free PMC article. Review.
References
- Pisetsky DS. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. - PubMed
- Hansen JD, Vojtech LN, Laing KJ. Sensing disease and danger: a survey of vertebrate PRRs and their origins. Dev Comp Immunol. 2011;35:886–897. - PubMed
- Ranjin P, Bowzard JB, Schwerzmann JW, Jeisy-Scott V, Fujita T, Sambhara S. Cytoplasmic nucleic acid sensors in antiviral immunity. Trends Mol Med. 2009;15:359–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources