Multiscale natural moves refine macromolecules using single-particle electron microscopy projection images - PubMed (original) (raw)
Multiscale natural moves refine macromolecules using single-particle electron microscopy projection images
Junjie Zhang et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The method presented here refines molecular conformations directly against projections of single particles measured by electron microscopy. By optimizing the orientation of the projection at the same time as the conformation, the method is well-suited to two-dimensional class averages from cryoelectron microscopy. Such direct use of two-dimensional images circumvents the need for a three-dimensional density map, which may be difficult to reconstruct from projections due to structural heterogeneity or preferred orientations of the sample on the grid. Our refinement protocol exploits Natural Move Monte Carlo to model a macromolecule as a small number of segments connected by flexible loops, on multiple scales. After tests on artificial data from lysozyme, we applied the method to the Methonococcus maripaludis chaperonin. We successfully refined its conformation from a closed-state initial model to an open-state final model using just one class-averaged projection. We also used Natural Moves to iteratively refine against heterogeneous projection images of Methonococcus maripaludis chaperonin in a mix of open and closed states. Our results suggest a general method for electron microscopy refinement specially suited to macromolecules with significant conformational flexibility. The algorithm is available in the program Methodologies for Optimization and Sampling In Computational Studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Obtaining the energy score of a model with an optimized orientation. The input orientation Ωin is the Euler angle used to project the model, X. I c is the centered target image, a constant parameter (step 1). A series of orientation angles Ω_k_ are generated around Ωin (step 2, see
SI Materials and Methods
). The model X is projected along these proposed orientations to generate the corresponding projection images I k (step 3). Negative cross-correlation scores  are calculated between these projection images and the image I c (step 4). The lowest value is assigned to the EM energy _E_EM between the model X and the input image I c (step 5). Its corresponding orientation is considered the optimized orientation Ωopt that projects X to fit I c. The outputs are the optimized projection orientation Ωopt and the total energy _E_total, which is the sum of a molecular energy, _E_mol and the EM energy _E_EM, weighted by an adjustable weight parameter w (step 6). _E_mol is a function of the current model X to ensure its proper stereochemistry.
are calculated between these projection images and the image I c (step 4). The lowest value is assigned to the EM energy _E_EM between the model X and the input image I c (step 5). Its corresponding orientation is considered the optimized orientation Ωopt that projects X to fit I c. The outputs are the optimized projection orientation Ωopt and the total energy _E_total, which is the sum of a molecular energy, _E_mol and the EM energy _E_EM, weighted by an adjustable weight parameter w (step 6). _E_mol is a function of the current model X to ensure its proper stereochemistry.
Fig. 2.
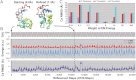
Temperature-modulated NM-MC refinement protocol with lysozyme as an example. A total of 2.03 million steps of temperature-modulated NM-MC were carried out for each refinement. No noise was added to the target 2D image projected from the target structure. (A) Each lysozyme model is represented by three rigid segments connected by two flexible loops (residues 40–42 and 85–88 drawn as spheres). (B) The temperature, T (brown), the energy (EM in red, total in blue), and C_α_ rmsd (purple) from the target lysozyme structure are shown as a function of the refinement steps. The initial model (A, Left) has an 8.6 Å C_α_ rmsd to the target. The model with the lowest EM energy (obtained at the step indicated by a black vertical dash line) is much closer to the target structure (1.3 Å C_α_ rmsd, A, Right). In this example, the EM image has no noise or orientation estimate error and the weight of the EM energy is 5. (C) The best C_α_ rmsd each refinement can achieve with eight different weights for the EM energy (from 0.01 to 100, as marked along the x axis) and three different orientation errors: 0° (blue bars), 4° (red bars), 8° (cyan bars). In the presence of orientation errors, the optimum weight value of 5 yields the lowest C_α_ rmsd values.
Fig. 3.
Lidless Mm-cpn model refined against a single cryo-EM projection class average. (A) Top view of the projection class-average image in the open state. Three subunits are labeled, and the projection orientation is estimated to be rotated by 22.5° in-plane relative to the initial model. (B) Schematic definition of the segments and their connecting loops (solid red lines) of lidless Mm-cpn showing the three subunits from (A). Neighboring subunits are colored grey and blue, respectively. The stem loop of one subunit is hydrogen bonded with the NC termini of the other, as indicated by dotted red lines. The viewing angle is from the eightfold symmetry axis. (C) Three levels of region compositions for a single subunit with hierarchically increasing DOF. (D) Top and side views of the initial model from PDB id 3J03 (blue), the refined model (orange), and the map-derived model from PDB id 3IYF (red). Eighty thousand temperature-modulated NM-MC steps were carried out successively for each of the three levels to refine the initial model against the 2D cryo-EM class average (
Fig. S3
and
Movies S1
–
S3
). The model with the lowest EM energy during the previous level of refinement was used as the initial model for the next level.
Fig. 4.
Separating heterogeneous conformations of lidless Mm-cpn with mixed states of EM particle images using the closed-state initial model. (A) Mixing 5000 ATP/aluminum fluoride-induced (closed) and 5000 ATP-free (open) states of lidless Mm-cpn raw particle images to generate the artificial heterogeneous EM dataset of 10,000 particles. (B) The 100 2D class averages that were generated from the 10,000 particle images. (C) Refinement results against six representative class averages exhibiting various conformational and orientational states such as open side view (Average #0), closed tilted view (Average #4), open top view (Average #30), closed side view (Average #51), closed top view (Average #88), and open tiled view (Average #96). Column I shows the projections of the initial closed-state model along the initially estimated orientations. Column C shows the target 2D experimental class averages. Column R shows the projection of each refined models (Column M) along the refined orientations. Column M shows the refined models after three iterations of NM-MC refinement (Materials and Methods). Note that for Average #30, the initially estimated orientation was not correct due to the large conformational difference between the initial model and the class average. After three iterations, both the correct orientation and conformation were obtained. (D) Seed maps generated from two clustered states of the refined models at 30 Å resolution (Left) and the re-refined maps at 9 Å resolution in which α-helices are visible (
SI Materials and Methods
,
Figs. S8
and
S9
).
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.
Pillay J, Gaudet LA, Saba S, Vandermeer B, Ashiq AR, Wingert A, Hartling L. Pillay J, et al. Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article. - Undernutrition as a risk factor for tuberculosis disease.
Franco JV, Bongaerts B, Metzendorf MI, Risso A, Guo Y, Peña Silva L, Boeckmann M, Schlesinger S, Damen JA, Richter B, Baddeley A, Bastard M, Carlqvist A, Garcia-Casal MN, Hemmingsen B, Mavhunga F, Manne-Goehler J, Viney K. Franco JV, et al. Cochrane Database Syst Rev. 2024 Jun 11;6(6):CD015890. doi: 10.1002/14651858.CD015890.pub2. Cochrane Database Syst Rev. 2024. PMID: 38860538 Free PMC article. Review. - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article.
Cited by
- Reconstruction of 3D structures of MET antibodies from electron microscopy 2D class averages.
Chen Q, Vieth M, Timm DE, Humblet C, Schneidman-Duhovny D, Chemmama IE, Sali A, Zeng W, Lu J, Liu L. Chen Q, et al. PLoS One. 2017 Apr 13;12(4):e0175758. doi: 10.1371/journal.pone.0175758. eCollection 2017. PLoS One. 2017. PMID: 28406969 Free PMC article. - Computational methods for constructing protein structure models from 3D electron microscopy maps.
Esquivel-Rodríguez J, Kihara D. Esquivel-Rodríguez J, et al. J Struct Biol. 2013 Oct;184(1):93-102. doi: 10.1016/j.jsb.2013.06.008. Epub 2013 Jun 21. J Struct Biol. 2013. PMID: 23796504 Free PMC article. - Structure of Ribosomal Silencing Factor Bound to Mycobacterium tuberculosis Ribosome.
Li X, Sun Q, Jiang C, Yang K, Hung LW, Zhang J, Sacchettini JC. Li X, et al. Structure. 2015 Oct 6;23(10):1858-1865. doi: 10.1016/j.str.2015.07.014. Epub 2015 Aug 20. Structure. 2015. PMID: 26299947 Free PMC article. - Assembly of macromolecular complexes by satisfaction of spatial restraints from electron microscopy images.
Velázquez-Muriel J, Lasker K, Russel D, Phillips J, Webb BM, Schneidman-Duhovny D, Sali A. Velázquez-Muriel J, et al. Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18821-6. doi: 10.1073/pnas.1216549109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112201 Free PMC article. - Massively parallel unsupervised single-particle cryo-EM data clustering via statistical manifold learning.
Wu J, Ma YB, Congdon C, Brett B, Chen S, Xu Y, Ouyang Q, Mao Y. Wu J, et al. PLoS One. 2017 Aug 7;12(8):e0182130. doi: 10.1371/journal.pone.0182130. eCollection 2017. PLoS One. 2017. PMID: 28786986 Free PMC article.
References
- Jiang W, et al. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–1134. - PubMed
- Ludtke SJ, et al. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources