IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques - PubMed (original) (raw)
IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques
H Xu et al. Mucosal Immunol. 2012 Nov.
Abstract
Innate lymphoid cells (ILCs) are an emerging subset of lymphocytes involved in surveillance against virally infected cells. Here, we show CD3(-)CD8(high) lymphocytes in macaque blood include major subsets of ILCs including natural killer (NK) cells expressing CD16, NKp46, and NKG2A, but also populations of ILCs in mucosal tissues having different properties. One ILC subset secreted interleukin (IL)-17 (ILC17), but these were restricted to mucosal tissues. Some mucosal ILC17 cells expressed classical NK-cell markers, but little NKG2A or NKG2D. Some ILC17 cells secreted IL-22 and tumor necrosis factor-α, but few produced interferon (IFN)-γ or contained granzyme B. IL-17 production by ILCs was induced by IL-6, transforming growth factor-β, and IL-23. Further, simian immunodeficiency virus (SIV) infection resulted in a significant loss of ILC17 cells, especially in the jejunum, which persisted throughout SIV infection. These findings indicate that ILC17 cells may be involved in innate mucosal immune responses, and their loss may contribute to loss of intestinal mucosal integrity and disease progression in human immunodeficiency virus (HIV)/SIV infection.
Figures
Figure 1. Phenotypic characterization of innate lymphoid cells in peripheral blood of normal rhesus macaques
(a) Representative gating strategy to define ILCs in PBMCs. Macaque ILCs were defined as CD3−CD8αhigh gated lymphocytes. (b) Representative dot plot depicting the expression of CD20, HLA-DR, CD14, CD11c, CD163 and CD11b+CD11int on CD3−CD8αhigh (R1) as compared to CD3−CD8αlow/− (R3) subsets. (c) Representative histogram showing expression of classical NK markers, CD16, CD56, NKp46, NKG2A and NKG2D on CD3−CD8αhigh (R1), CD3+CD8+ (R2), CD3-CD8−/intermediate (R3), and CD3+CD4+ (R4) subsets from peripheral blood. (d) Expression of classical NK markers on CD3−CD8αhigh (R1), CD3+CD8+ (R2), CD3-CD8−/intermediate (R3), and CD3+CD4+ (R4) subsets from peripheral blood (n=8). Examples are representative of 8 naive animals. *, p<0.001; **, p<0.05, compared between CD3−CD8high and other three subsets, respectively.
Figure 2. Comparison of anti-CD16 antibody clones for cross-reactivity with rhesus macaques, and distribution of NK cells in lymphoid tissues
(a) Cross-reactivity and comparison of whole blood (WB) and washed PBMC from the same animals using different anti-CD16 antibody clones in chronically SIV infected rhesus macaques (n=8). Note only the DJ130c clone does not demonstrate differential staining between washed PBMC and WB. (b) Tissue distribution of classical NK cell subsets (CD16+, CD56+, NKG2A+) in naïve rhesus macaques (n=5). *, p<0.05 between using 3G8 and DJ130c clones in PBMC samples (a) or blood compared with other tissues (b); **, p<0.01 between using 3G8 and DJ130c clones in whole blood; #, p<0.01, compared with peripheral blood; ##. p<0.05 between liver and other tissues (except blood), respectively.
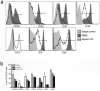
Figure 3. Distinct expression of surface molecules on innate lymphoid cells between mucosal tissues and peripheral blood
(a) Representative histogram displaying different expression of molecules on ILCs from PBMCs and jejunum lamina propria. (b) Statistical comparison of expression between ILCs from PBMCs and jejunum lamina propria in naïve rhesus macaques (n=12). *, p<0.05.
Figure 4. Innate lymphoid cells that secrete IL-17 are restricted to mucosal tissues in rhesus macaques
(a) Jejunum dot plots gated on IL-17-secreting lymphocytes (left) contain both CD3+ and CD3− subsets in jejunum after PMA/Ionomycin stimulation. (b) Comparison of IL-17-producing CD4+ (Th17), CD8+ T cells, and CD3−CD8high (ILCs) in various lymphoid tissues, including peripheral blood, jejunum, colon, spleen, tonsil and duodenum. Noted that IL-17-secreting ILCs are restricted to mucosal tissues, with little to no expression of IL-17 from PBMC or spleen-derived ILCs.
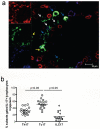
Figure 5. Detection of ILC17 in jejunum tissues of normal rhesus macaques by confocal microscopy
(a) Jejunum from a normal macaque showing CD3+ (red), IL-17 (green) and CD8+ (blue) cells. The white arrows show ILC17 cells (CD3−CD8+IL-17+); the yellow arrows demonstrate Th17 cells (CD3+CD8−IL-17+), and the blue arrows show Tc17 cells (CD3+CD8+IL-17+). Scale bar = 10 μm. (b) Relative percentages of Th17 , Tc17, and ILC17 cells gated through total IL-17+ lymphocytes in the jejunum of normal animals as assessed by flow cytometry.
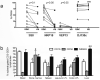
Figure 6. Phenotyping IL-17-producing ILCs for classical NK cell markers and cytokine secretion in peripheral blood and mucosal tissues of normal rhesus macaques
(a) Expression of classical NK cell markers on ILC17 cells in the peripheral blood, jejunum and colon. Note there was little to no production of IL-17 from NKG2A+, or NKG2D+ ILCs. (b) Cytokine secretion of ILC17 cells isolated from jejunum after PMA/ionomycin stimulation. Note ILC17 cells also secrete pro-inflammatory (TNF-α), and innate (IL-22) cytokines, but express little to no IFN-γ or Granzyme B. (c) Response of jejunum ILC17 cells to various cytokine and LPS stimulation.
Figure 7. Effects of SIV infection on blood and jejunum-derived innate lymphoid cells (CD3−CD8high) or its subpopulations in rhesus macaques
(a and b) Prospective analysis of changes in percentage (a) and absolute numbers (b) of total CD3−CD8high ILCs in peripheral blood after SIV infection. (c, e–g, and i–k) Dynamic of ILCs and their subpopulation in blood and jejunum ILCs after SIV infection (Naïve, n=55; 7dpi, n=30; 14dpi, n=23; 21dpi, n=26; 28dpi, n=26; 35dpi, n=18; chronic, n=23). (d, h and i) Proliferation (Ki-67+) of circulating or intestinal ILCs after SIV infection (Naïve, n=24; 7dpi, n=5; 14dpi, n=5; 21dpi, n=4; 28dpi, n=3; 42dpi, n=4; chronic, n=14). *, p<0.05, compared with uninfected normal animals.
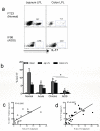
Figure 8. Reduction of intestinal mucosal ILC17 cells after SIV infection of rhesus macaques
(a) Dot plot demonstrating ILC17 cells after gating on CD3−CD8high subsets from the jejunum and colon from a representative normal and AIDS macaque. (b) Loss of intestinal ILCs occurs after SIV infection (Normal, n=10; acute, n=6; chronic, 12; AIDS, n=4). *, P<0.05. (c) Correlation between percentages of ILC17 cells and CD4+ T cells or (d) Th17 cells in jejunum during SIV infection (c, n=18; d, n=28).
Similar articles
- Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection.
Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM. Klatt NR, et al. Mucosal Immunol. 2012 Nov;5(6):646-57. doi: 10.1038/mi.2012.38. Epub 2012 May 30. Mucosal Immunol. 2012. PMID: 22643849 Free PMC article. - Presence of Inflammatory Group I and III Innate Lymphoid Cells in the Colon of Simian Immunodeficiency Virus-Infected Rhesus Macaques.
Cogswell A, Ferguson N, Barker E. Cogswell A, et al. J Virol. 2020 Apr 16;94(9):e01914-19. doi: 10.1128/JVI.01914-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32051277 Free PMC article. - Functional Perturbation of Mucosal Group 3 Innate Lymphoid and Natural Killer Cells in Simian-Human Immunodeficiency Virus/Simian Immunodeficiency Virus-Infected Infant Rhesus Macaques.
Hueber B, Curtis AD 2nd, Kroll K, Varner V, Jones R, Pathak S, Lifton M, Van Rompay KKA, De Paris K, Reeves RK. Hueber B, et al. J Virol. 2020 Feb 14;94(5):e01644-19. doi: 10.1128/JVI.01644-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801861 Free PMC article. - Monkeying Around: Using Non-human Primate Models to Study NK Cell Biology in HIV Infections.
Manickam C, Shah SV, Nohara J, Ferrari G, Reeves RK. Manickam C, et al. Front Immunol. 2019 May 22;10:1124. doi: 10.3389/fimmu.2019.01124. eCollection 2019. Front Immunol. 2019. PMID: 31191520 Free PMC article. Review. - Adaptive NK cell responses in HIV/SIV infections: A roadmap to cell-based therapeutics?
Ram DR, Manickam C, Lucar O, Shah SV, Reeves RK. Ram DR, et al. J Leukoc Biol. 2019 Jun;105(6):1253-1259. doi: 10.1002/JLB.MR0718-303R. Epub 2019 Feb 7. J Leukoc Biol. 2019. PMID: 30730588 Free PMC article. Review.
Cited by
- Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease.
Sonnenberg GF, Artis D. Sonnenberg GF, et al. Immunity. 2012 Oct 19;37(4):601-10. doi: 10.1016/j.immuni.2012.10.003. Immunity. 2012. PMID: 23084357 Free PMC article. Review. - Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression.
Mudd JC, Brenchley JM. Mudd JC, et al. J Infect Dis. 2016 Oct 1;214 Suppl 2(Suppl 2):S58-66. doi: 10.1093/infdis/jiw258. J Infect Dis. 2016. PMID: 27625432 Free PMC article. - Mucosal immunity in human and simian immunodeficiency lentivirus infections.
Brenchley JM. Brenchley JM. Mucosal Immunol. 2013 Jul;6(4):657-65. doi: 10.1038/mi.2013.15. Epub 2013 Apr 3. Mucosal Immunol. 2013. PMID: 23549448 Free PMC article. Review. - Learning to Be Elite: Lessons From HIV-1 Controllers and Animal Models on Trained Innate Immunity and Virus Suppression.
Sugawara S, Reeves RK, Jost S. Sugawara S, et al. Front Immunol. 2022 Apr 27;13:858383. doi: 10.3389/fimmu.2022.858383. eCollection 2022. Front Immunol. 2022. PMID: 35572502 Free PMC article. Review. - Infection and depletion of CD4+ group-1 innate lymphoid cells by HIV-1 via type-I interferon pathway.
Zhao J, Cheng L, Wang H, Yu H, Tu B, Fu Q, Li G, Wang Q, Sun Y, Zhang X, Liu Z, Chen W, Zhang L, Su L, Zhang Z. Zhao J, et al. PLoS Pathog. 2018 Jan 5;14(1):e1006819. doi: 10.1371/journal.ppat.1006819. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29304123 Free PMC article.
References
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. - PubMed
- Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33(5):752–764. - PubMed
- Berger CT, Alter G. Natural killer cells in spontaneous control of HIV infection. Curr Opin HIV AIDS. 2011;6(3):208–213. - PubMed
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15(1):45–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI49080/AI/NIAID NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- R01 AI084793/AI/NIAID NIH HHS/United States
- RR000164/RR/NCRR NIH HHS/United States
- R01 AI049080/AI/NIAID NIH HHS/United States
- AI084793/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous