Quantitative proteomics profiling of the poly(ADP-ribose)-related response to genotoxic stress - PubMed (original) (raw)
Quantitative proteomics profiling of the poly(ADP-ribose)-related response to genotoxic stress
Jean-Philippe Gagné et al. Nucleic Acids Res. 2012 Sep.
Abstract
Upon DNA damage induction, DNA-dependent poly(ADP-ribose) polymerases (PARPs) synthesize an anionic poly(ADP-ribose) (pADPr) scaffold to which several proteins bind with the subsequent formation of pADPr-associated multiprotein complexes. We have used a combination of affinity-purification methods and proteomics approaches to isolate these complexes and assess protein dynamics with respect to pADPr metabolism. As a first approach, we developed a substrate trapping strategy by which we demonstrate that a catalytically inactive Poly(ADP-ribose) glycohydrolase (PARG) mutant can act as a physiologically selective bait for the isolation of specific pADPr-binding proteins through its macrodomain-like domain. In addition to antibody-mediated affinity-purification methods, we used a pADPr macrodomain affinity resin to recover pADPr-binding proteins and their complexes. Second, we designed a time course experiment to explore the changes in the composition of pADPr-containing multiprotein complexes in response to alkylating DNA damage-mediated PARP activation. Spectral count clustering based on GeLC-MS/MS analysis was complemented with further analyses using high precision quantitative proteomics through isobaric tag for relative and absolute quantitation (iTRAQ)- and Stable isotope labeling by amino acids in cell culture (SILAC)-based proteomics. Here, we present a valuable resource in the interpretation of systems biology of the DNA damage response network in the context of poly(ADP-ribosyl)ation and provide a basis for subsequent investigations of pADPr-binding protein candidates.
Figures
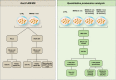
Figure 1.
Schematic representation of the experimental design and proteomics strategies to identify pADPr-associated protein complexes. A combination of affinity-purification procedures coupled with MS was used to generate a global protein profile of pADPr-associated protein complexes (GeLC-MS/MS—left panel). Proteomics strategies that integrate relative quantitation with affinity-purification MS were used to provide a time-resolved proteome profile of protein networks responsive to pADPr turnover (right panel). Complementary label-free and label-based quantitative proteomics approaches were used to identify and evaluate protein changes occurring in cells following alkylation-induced DNA damage and PARP activation. 10H IPs: Immunoprecipitations with anti-pADPr antibody clone 10H; PARG-DEAD IPs: IP of catalytically inactive PARG, as described in the text.
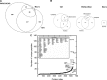
Figure 2.
Diversity of pADPr-associated proteins as revealed by gel-based LC-MS/MS analysis. Complementary proteomic approaches directed towards identification of novel proteins that interact with pADPr were integrated to mine the accessible pADPr-binding interactome. IPs were performed directly against pADPr using a high affinity monoclonal antibody (clone 10H) or indirectly by a novel pADPr substrate trapping approach targeting a catalytically inactive PARG mutant and a macrodomain protein (see text for details). (A) The area-proportional Venn diagram shows unique and shared protein identifications in pADPr-associated protein datasets that originate from each strategies. (B) Area-proportional Venn diagrams depicting the distribution of proteins in subcellular compartments for each datasets. Proteins were classified into cytoplasmic, nuclear or mitochondrial compartments according to GO classification. (C) Classification of pADPr-associated proteins. Proteins are ordered relative to the number of unique peptides assigned. The inner frame lists some DNA damage response factors and chromatin-associated proteins with their corresponding number of unique peptide assignments. Refer to
Supplementary Table S1
for detailed protein listing.
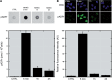
Figure 3.
pADPr dynamics following MNNG-induced DNA damage and PARP activation. (A) Dot-blot analysis of pADPr levels in pADPr IP extracts from MNNG-treated HEK 293 cells. Cellular material bound to 10H-coupled magnetic beads was eluted and hand-blotted on positively charged nylon membrane. pADPr was detected using 96-10 antibody (upper panel). pADPr signals in IP extracts were quantified using DHBB-purified pADPr as a reference value for quantitation and displayed on a bar graph (lower panel). The data are represented as the mean ± SEM (n = 4). (B) The 10H immunofluorescence labeling of pADPr in HEK 293 cells exposed to MNNG (upper panel). Confocal fluorescent images were obtained by a Zeiss LSM 510 NLO laser scanning confocal microscope. A region was drawn inside of each nucleus (n = 100) to establish the mean fluorescence intensity. Relative pADPr levels were plotted on a bar graph (lower panel) and displayed as the mean ± SEM (n = 3).
Figure 4.
Correlated accumulation of DNA damage response factors with pADPr. (A) The 10H-based IPs using HEK 293 whole cell extracts were performed to isolate pADPr-associated proteins in the context of MNNG-induced DNA damage and PARP activation. Cells were allowed to recover from MNNG by incubation with fresh medium and IPs were performed at the indicated times. Undamaged control cells were pre-incubated 2 h with 5-µM PARP-1 inhibitor ABT-888 before lysis. Several DNA damage response factors were screened for entrapment in anti-pADPr IP extracts. Cell lysates (inputs) were also subjected to western blot analysis using the corresponding antibodies. (B) pADPr levels correlate with the accumulation of several DNA damage response factors involved in major DNA repair pathways. Relative quantitation of western blot signal intensities shown in (A) were measured and expressed relative to control protein levels. A greyscale heatmap ranks each of the protein accumulation ratios.
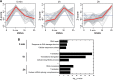
Figure 5.
Protein abundance profiles in time-resolved pADPr IPs. Spectral counting-based quantitation was combined with Scaffold’s protein validation tools to provide a quantitative protein profile. pADPr-associated proteins identified by GeLC-MS/MS in IP extracts were grouped by _K_-means clustering for each treatment, respectively. (A) The kinetics of protein accumulation is displayed by trend curves showing the overlay of the proteins grouped by each cluster. The red line represents the mean value at each time-point for all the proteins in the cluster. (B) Protein clusters were searched for significant over-representation of proteins belonging to specific pathways according to the GO database using DAVID. Bar plots of the most significant biological processes in each datasets are shown. The significance of the enrichment is expressed as a function of the _P_-value, which indicates whether a biological process is significantly higher than random expectations. Refer to
Supplementary Table S2
for complete protein listing.
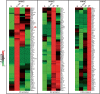
Figure 6.
Heatmap analysis with _K_-means clustering. Temporal profiling of pADPr-associated proteins in HEK 293 cells upon MNNG exposure was performed based on the GeLC-MS/MS spectral count quantitation. The heatmap displays the three clusters identified by the _K_-means algorithm that correspond to the time-points analyzed after MNNG exposure. Green indicates the lowest ratio, black indicates an intermediate value and red indicates the highest ratio (protein enrichment). Proteins in each cluster are listed according to their gene symbol. A red arrow indicates the presence of PARP-1.
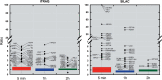
Figure 7.
Box plot statistics to define outlier significance for iTRAQ and SILAC analysis. pADPr IPs were carried out after each of the three time-points examined following MNNG exposure. Protein isolates were quantified with respect to basal levels of pADPr in control IPs. Each box encloses 50% of the data with the median value of the variable displayed as a line. The top and bottom of the box mark the limits of upper and lower quartiles. The vertical lines extending from the top and bottom of each box mark the minimum and maximum values within the data set that fall within an acceptable range (1.5 × interquartile distance). Any value outside of this range (outlier) is displayed as an individual point with the corresponding gene symbol. Refer to
Supplementary Table S3
(iTRAQ) and
Supplementary Table S4
(SILAC) for detailed protein annotations.
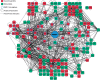
Figure 8.
Subnetwork diagram of the PARP-1-centered protein interaction map. Cytoscape was used to construct a global network of the pADPr-associated proteome that integrates protein identification from all the proteomics approaches that have been carried out in this study. The diagram shown consists of the nearest-neighbors subnetwork of PARP family members in addition to selected proteins from DNA damage response pathways (See text for details). The subnetwork emphasizes the pADPr-associated protein regulatory network centered around PARP-1 in cellular recovery to DNA damage. The red coloring indicates top-scoring proteins and refers to predominant proteins in either of the four datasets (GeLC-MS/MS, Spectral count, iTRAQ, SILAC). Interactions among proteins are reported. The network comprises 164 proteins (nodes) and 899 interactions (edges). Refer to
Supplementary Table S5
for complete protein listing.
Figure 9.
Dynamics of DNA damage response proteins at laser-induced DNA breaks. DNA damage induced by laser micro-irradiation in subnuclear region of single living cells was performed to evaluate the pADPr-dependent recruitment of DNA repair factors at DNA damage sites. (A) Schematic representation of the micro-irradiation system used to introduce DNA lesions. (B) Local accumulation of DNA repair factors at laser-induced DNA damage sites. A 750-nm two-photon laser beam was focused on Hoechst-sensitized cells and the accumulation of GFP-tagged DNA repair factors was monitored on a Zeiss LSM 510 NLO laser scanning confocal microscope. (C) Evaluation of the contribution of pADPr to the recruitment kinetics of DNA damage response factors at sites of DNA damage. The dynamics of several GFP-tagged proteins involved in DNA repair pathways were analyzed in the context of PARP inhibition (ABT-888). The HEK 293 cells transiently expressing the targeted proteins were sensitized with Hoechst 33342 and micro-irradiated with femtosecond near-infrared (750-nm) pulses from a Ti:sapphire laser. The intensity of fluorescence was recorded on a Zeiss LSM 510 NLO laser-scanning confocal microscope. The dynamics of DNA repair factors under normal conditions was compared with the dynamics observed following PARP inhibition with 5 µM ABT-888. Targeted proteins involved in BER (XRCC1, LIG3, FEN1), NHEJ (KU70, KU80, LIG4, XRCC4, XLF, ARTEMIS) and chromatin remodeling (CHFR, SSRP1) are displayed. Because of the rapid accumulation of DNA repair proteins at DNA damage sites, multiple acquisition rates were used (see ‘Materials and Methods’ section). Background and photobleaching corrections were applied to each dataset. A minimum of eight recruitments per construct were collected and analyzed. The error bars represent the SEM.
Similar articles
- Quantitative proteomics and dynamic imaging reveal that G3BP-mediated stress granule assembly is poly(ADP-ribose)-dependent following exposure to MNNG-induced DNA alkylation.
Isabelle M, Gagné JP, Gallouzi IE, Poirier GG. Isabelle M, et al. J Cell Sci. 2012 Oct 1;125(Pt 19):4555-66. doi: 10.1242/jcs.106963. Epub 2012 Jul 5. J Cell Sci. 2012. PMID: 22767504 - Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes.
Gagné JP, Isabelle M, Lo KS, Bourassa S, Hendzel MJ, Dawson VL, Dawson TM, Poirier GG. Gagné JP, et al. Nucleic Acids Res. 2008 Dec;36(22):6959-76. doi: 10.1093/nar/gkn771. Epub 2008 Nov 3. Nucleic Acids Res. 2008. PMID: 18981049 Free PMC article. - Reprogramming cellular events by poly(ADP-ribose)-binding proteins.
Krietsch J, Rouleau M, Pic É, Ethier C, Dawson TM, Dawson VL, Masson JY, Poirier GG, Gagné JP. Krietsch J, et al. Mol Aspects Med. 2013 Dec;34(6):1066-87. doi: 10.1016/j.mam.2012.12.005. Epub 2012 Dec 23. Mol Aspects Med. 2013. PMID: 23268355 Free PMC article. Review. - Methods for purification of proteins associated with cellular poly(ADP-ribose) and PARP-specific poly(ADP-ribose).
Rood JE, Leung AK, Chang P. Rood JE, et al. Methods Mol Biol. 2011;780:153-64. doi: 10.1007/978-1-61779-270-0_10. Methods Mol Biol. 2011. PMID: 21870260 Free PMC article. - Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism.
Davidovic L, Vodenicharov M, Affar EB, Poirier GG. Davidovic L, et al. Exp Cell Res. 2001 Aug 1;268(1):7-13. doi: 10.1006/excr.2001.5263. Exp Cell Res. 2001. PMID: 11461113 Review.
Cited by
- Systematic prediction of degrons and E3 ubiquitin ligase binding via deep learning.
Hou C, Li Y, Wang M, Wu H, Li T. Hou C, et al. BMC Biol. 2022 Jul 14;20(1):162. doi: 10.1186/s12915-022-01364-6. BMC Biol. 2022. PMID: 35836176 Free PMC article. - Glioblastoma cells containing mutations in the cohesin component STAG2 are sensitive to PARP inhibition.
Bailey ML, O'Neil NJ, van Pel DM, Solomon DA, Waldman T, Hieter P. Bailey ML, et al. Mol Cancer Ther. 2014 Mar;13(3):724-32. doi: 10.1158/1535-7163.MCT-13-0749. Epub 2013 Dec 19. Mol Cancer Ther. 2014. PMID: 24356817 Free PMC article. - Proteomics approaches to identify mono-(ADP-ribosyl)ated and poly(ADP-ribosyl)ated proteins.
Vivelo CA, Leung AK. Vivelo CA, et al. Proteomics. 2015 Jan;15(2-3):203-17. doi: 10.1002/pmic.201400217. Epub 2014 Dec 15. Proteomics. 2015. PMID: 25263235 Free PMC article. - Poly(ADP-ribose) polymerase inhibitors sensitize cancer cells to death receptor-mediated apoptosis by enhancing death receptor expression.
Meng XW, Koh BD, Zhang JS, Flatten KS, Schneider PA, Billadeau DD, Hess AD, Smith BD, Karp JE, Kaufmann SH. Meng XW, et al. J Biol Chem. 2014 Jul 25;289(30):20543-58. doi: 10.1074/jbc.M114.549220. J Biol Chem. 2014. PMID: 24895135 Free PMC article. - Coordinated regulation of plant immunity by poly(ADP-ribosyl)ation and K63-linked ubiquitination.
Yao D, Arguez MA, He P, Bent AF, Song J. Yao D, et al. Mol Plant. 2021 Dec 6;14(12):2088-2103. doi: 10.1016/j.molp.2021.08.013. Epub 2021 Aug 18. Mol Plant. 2021. PMID: 34418551 Free PMC article.
References
- Tartier L, Spenlehauer C, Newman HC, Folkard M, Prise KM, Michael BD, Menissier-de Murcia J, de Murcia G. Local DNA damage by proton microbeam irradiation induces poly(ADP-ribose) synthesis in mammalian cells. Mutagenesis. 2003;18:411–416. - PubMed
- Rouleau M, Aubin RA, Poirier GG. Poly(ADP-ribosyl)ated chromatin domains: access granted. J. Cell Sci. 2004;117:815–825. - PubMed
- Malanga M, Althaus FR. The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem. Cell Biol. 2005;83:354–364. - PubMed
- Till S, Ladurner AG. Sensing NAD metabolites through macro domains. Front. Biosci. 2009;14:3246–3258. - PubMed
- Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, West SC. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials