Fundamentals of FGF19 & FGF21 action in vitro and in vivo - PubMed (original) (raw)
Fundamentals of FGF19 & FGF21 action in vitro and in vivo
Andrew C Adams et al. PLoS One. 2012.
Abstract
Fibroblast growth factors 19 (FGF19) and 21 (FGF21) have emerged as key regulators of energy metabolism. Several studies have been conducted to understand the mechanism of FGF19 and FGF21 action, however, the data presented has often been inconsistent and at times contradictory. Here in a single study we compare the mechanisms mediating FGF19/FGF21 actions, and how similarities/differences in actions at the cellular level between these two factors translate to common/divergent physiological outputs. Firstly, we show that in cell culture FGF19/FGF21 are very similar, however, key differences are still observed differentiating the two. In vitro we found that both FGF's activate FGFRs in the context of βKlotho (KLB) expression. Furthermore, both factors alter ERK phosphorylation and glucose uptake with comparable potency. Combination treatment of cells with both factors did not have additive effects and treatment with a competitive inhibitor, the FGF21 delta N17 mutant, also blocked FGF19's effects, suggestive of a shared receptor activation mechanism. The key differences between FGF21/FGF19 were noted at the receptor interaction level, specifically the unique ability of FGF19 to bind/signal directly via FGFR4. To determine if differential effects on energy homeostasis and hepatic mitogenicity exist we treated DIO and ob/ob mice with FGF19/FGF21. We find comparable efficacy of the two proteins to correct body weight and serum glucose in both DIO and ob/ob mice. Nevertheless, FGF21 and FGF19 had distinctly different effects on proliferation in the liver. Interestingly, in vivo blockade of FGF21 signaling in mice using ΔN17 caused profound changes in glycemia indicative of the critical role KLB and FGF21 play in the regulation of glucose homeostasis. Overall, our data demonstrate that while subtle differences exist in vitro the metabolic effects in vivo of FGF19/FGF21 are indistinguishable, supporting a shared mechanism of action for these two hormones in the regulation of energy balance.
Conflict of interest statement
Competing Interests: All authors of this manuscript were employed by Eli Lilly and Company, Indianapolis, Indiana. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
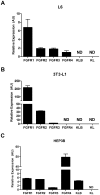
Figure 1. Expression of FGF receptors and Klotho co factors in cell culture models.
In 3T3-L1 cells we found a high level of FGFR1 expression along with modest levels of FGFR2 and FGFR3. In these cells FGFR4, KL and KLB were not detectable (A). In Hep3B cells there was detectable expression of all 4 FGF receptor subtypes, however, we detected especially high levels of FGFR4. Hep3B cells were also found have appreciable expression of KLB while KL was not detectable (B). In L6 cells expression of all FGFRs was extremely low in comparison to other cells lines we screened in addition to undetectable levels of KLB at baseline (C).
Figure 2. The “hormone like” FGFs exhibit different signaling properties in vitro.
Panel 1. In 3T3-L1 fibroblasts over-expressing KL we saw phosphorylation of the FGF target ERK upon treatment with FGF23, while FGF19 and 21 had no effect (A). Conversely, in 3T3-L1/KLB cells we saw no effect of FGF23 but potent signaling with FGF19 or FGF21 treatment (B). When we examined glucose uptake in the 3T3-L1/KL cells we found that only FGF23 lead to its stimulation (C). As we saw with pERK in 3T3-L1/KLB cells FGF19 and FGF21 both increased glucose uptake significantly (D). In 3T3-L1 fibroblasts expressing FGFR4 in the absence of KLB only FGF19 was able to increase glucose uptake (E). Furthermore, in Hep3B cells which show a relative enrichment of FGFR4, FGF19 was significantly more potent than FGF21 in inducing expression of the immediate early gene EGR1 (F). When 3T3-L1 cells were differentiated to become mature adipocytes FGF19 was also more potent than FGF21 even in the absence of FGFR4 expression suggesting a possible unknown factor which is not present on fibroblasts may be affecting FGF19's action in these cells, or vice versa (G). In 3T3-L1 adipocytes treated with either FGF19, FGF21 or a combination of both we did not see any additive or synergistic effects of combination treatment over individual therapy suggesting FGF19 and FGF21 share a common mechanism of action (H). To confirm our initial results regarding the specificity of FGF19 for FGFR4 we turned to L6 cells which have been reported to have extremely low expression of FGFRs and KLs. In parental L6 cells treatment with FGF19 had no effect on the level of ERK phosphorylation, however, when cells were transfected with KLB a small but significant increase in pERK was detected. Furthermore, when FGFR4 was added the response to FGF19 stimulation was magnified. Interestingly, we saw again that in cells transfected with FGFR4 alone FGF19 was also able to induce pERK confirming KLB independent signaling with this ligand can occur (I). In contrast to FGF19, cells treated with FGF21 showed pERK induction only in the presence of KLB with the level of this baseline induction similar to that seen with FGF19 treatment (J).
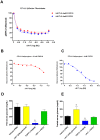
Figure 3. Inhibition of FGF21 signaling also blocks FGF19 action in vitro.
Panel 1. In 3T3-L1/KLB fibroblasts co-treatment with the competitive agonist ΔN17 was able to block the induction of ERK phosphorylation caused not only by FGF21 but also by FGF19 (A). In our glucose uptake assay in 3T3-L1 adipocytes ΔN17 also suppressed the activity of both FGF19 (B) and FGF21 (C). Panel 2. To determine if the inhibition of FGF19/21 signaling we see in vitro translates to effects on metabolic parameters in vivo we examined fed and fasted glucose levels in ob/ob mice treated with FGF21, ΔN17 or a combination of both. In fed mice treatment with ΔN17 alone had no effect on serum glucose. FGF21 treatment reduced glucose levels significantly in the fed state, however, when the two treatments are combined the effect of FGF21 to reduce glucose is abolished (D). In fasted animals FGF21 again reduced glucose, an effect blocked by combination with ΔN17. Interestingly, we found that in fasted animals treatment with ΔN17 partially blocked the normal reduction in the serum glucose, suggesting ΔN17 may interfere in the regulation of glucose homeostasis (E).
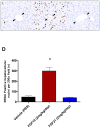
Figure 4. FGF19 treatment leads to hepatocellular proliferation.
All mice received a constant infusion of BRDU during the 7 days of treatment. At the end of treatment samples of liver were harvested, preserved in formalin, processed routinely, and embedded in paraffin. Tissue sections were cut, immunolabeled for BRDU, and counterstained with hematoxylin. Representative sections are shown here from mice receiving injections of phosphate buffered saline (PBS; A), FGF19 (B), or FGF21 (C). The average number of BRDU-positive nuclei per 200× microscopic field is shown. Mice administered FGF19 had a statistically significant increase (* = p<0.0001) in the numbers of hepatocytes with BRDU-positive nuclei when compared to mice administered PBS (D). In contrast to FGF19, FGF21 did not induce hepatocellular proliferation. The arrowheads indicate the location of the centrilobular veins.
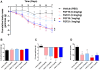
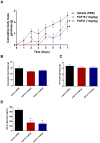
Figure 5. Treatment of DIO mice with either FGF19 or FGF21 improves metabolic dysfunction.
Administration of recombinant FGF19 or FGF21 led to a reduction in body mass in a dose dependent fashion (A). Both FGF19 and FGF21 therapy led to a trend towards increased food intake, however, these differences were not significant (B). Treatment with FGF19 or FGF21 significantly reduced adiposity at both doses tested again in a dose dependant manner (C), All FGF treatments caused a significant reduction in serum glucose in DIO animals, furthermore, the reduction in glucose observed with the two proteins was strikingly similar (D).
Figure 6. Treatment of ob/ob mice with either FGF19 or FGF21 improves metabolic dysfunction.
In ob/ob mice neither FGF19 nor FGF21 were able to reduce body mass significantly; however, both treatment groups exhibited significant reductions in body mass accrual over the 7 day treatment period (A). Food intake was significantly reduced in the FGF19 treated mice while FGF21 treatment caused a trend to reduced caloric intake (B). No difference in adipose mass was observer following administration of either FGF19 or FGF21 (C) While the ob/ob mice showed significantly less profound effects on body mass and adiposity than was observed in the DIO group the glucose lowering following treatment with either FGF19 or FGF21 was still very significant suggesting possible partitioning of the effects of the endocrine FGFs (D).
Similar articles
- Molecular elements in FGF19 and FGF21 defining KLB/FGFR activity and specificity.
Agrawal A, Parlee S, Perez-Tilve D, Li P, Pan J, Mroz PA, Kruse Hansen AM, Andersen B, Finan B, Kharitonenkov A, DiMarchi RD. Agrawal A, et al. Mol Metab. 2018 Jul;13:45-55. doi: 10.1016/j.molmet.2018.05.003. Epub 2018 May 11. Mol Metab. 2018. PMID: 29789271 Free PMC article. - Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB.
Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Yang C, et al. PLoS One. 2012;7(3):e33870. doi: 10.1371/journal.pone.0033870. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442730 Free PMC article. - Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21.
Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-O M. Kurosu H, et al. J Biol Chem. 2007 Sep 14;282(37):26687-26695. doi: 10.1074/jbc.M704165200. Epub 2007 Jul 10. J Biol Chem. 2007. PMID: 17623664 Free PMC article. - FGF19 and FGF21 for the Treatment of NASH-Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human.
Henriksson E, Andersen B. Henriksson E, et al. Front Endocrinol (Lausanne). 2020 Dec 22;11:601349. doi: 10.3389/fendo.2020.601349. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33414764 Free PMC article. Review. - MicroRNA-34a and Impaired FGF19/21 Signaling in Obesity.
Fu T, Kemper JK. Fu T, et al. Vitam Horm. 2016;101:175-96. doi: 10.1016/bs.vh.2016.02.002. Epub 2016 Mar 21. Vitam Horm. 2016. PMID: 27125742 Free PMC article. Review.
Cited by
- Serum FGF21 levels are associated with brown adipose tissue activity in humans.
Hanssen MJ, Broeders E, Samms RJ, Vosselman MJ, van der Lans AA, Cheng CC, Adams AC, van Marken Lichtenbelt WD, Schrauwen P. Hanssen MJ, et al. Sci Rep. 2015 May 18;5:10275. doi: 10.1038/srep10275. Sci Rep. 2015. PMID: 25985218 Free PMC article. - The anti-obesity effect of FGF19 does not require UCP1-dependent thermogenesis.
Antonellis PJ, Droz BA, Cosgrove R, O'Farrell LS, Coskun T, Perfield JW 2nd, Bauer S, Wade M, Chouinard TE, Brozinick JT, Adams AC, Samms RJ. Antonellis PJ, et al. Mol Metab. 2019 Dec;30:131-139. doi: 10.1016/j.molmet.2019.09.006. Epub 2019 Sep 29. Mol Metab. 2019. PMID: 31767164 Free PMC article. - The Origins, Evolution, and Future of Dietary Methionine Restriction.
Fang H, Stone KP, Wanders D, Forney LA, Gettys TW. Fang H, et al. Annu Rev Nutr. 2022 Aug 22;42:201-226. doi: 10.1146/annurev-nutr-062320-111849. Epub 2022 May 19. Annu Rev Nutr. 2022. PMID: 35588443 Free PMC article. Review. - Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21.
Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Li Y, et al. Gastroenterology. 2014 Feb;146(2):539-49.e7. doi: 10.1053/j.gastro.2013.10.059. Epub 2013 Nov 1. Gastroenterology. 2014. PMID: 24184811 Free PMC article. - FGF21 requires βklotho to act in vivo.
Adams AC, Cheng CC, Coskun T, Kharitonenkov A. Adams AC, et al. PLoS One. 2012;7(11):e49977. doi: 10.1371/journal.pone.0049977. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209629 Free PMC article.
References
- Yeoh JS, de Haan G. Fibroblast growth factors as regulators of stem cell self-renewal and aging. Mech Ageing Dev. 2007;128:17–24. - PubMed
- Chen GJ, Forough R. Fibroblast growth factors, fibroblast growth factor receptors, diseases, and drugs. Recent Pat Cardiovasc Drug Discov. 2006;1:211–224. - PubMed
- Kharitonenkov A. FGFs and metabolism. Curr Opin Pharmacol. 2009;9:805–810. - PubMed
- Kurosu H, Kuro-o M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17:368–372. - PubMed
- Wu X, Ge H, Gupte J, Weiszmann J, Shimamoto G, et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem. 2007;282:29069–29072. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous