Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors - PubMed (original) (raw)
. 2012 Jun 15;26(12):1326-38.
doi: 10.1101/gad.191056.112. Epub 2012 Jun 7.
Hui Yang, Wei Xu, Shenghong Ma, Huaipeng Lin, Honguang Zhu, Lixia Liu, Ying Liu, Chen Yang, Yanhui Xu, Shimin Zhao, Dan Ye, Yue Xiong, Kun-Liang Guan
Affiliations
- PMID: 22677546
- PMCID: PMC3387660
- DOI: 10.1101/gad.191056.112
Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors
Mengtao Xiao et al. Genes Dev. 2012.
Erratum in
- Corrigendum: inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors.
Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan KL. Xiao M, et al. Genes Dev. 2015 Apr 15;29(8):887. Epub 2015 Apr 15. Genes Dev. 2015. PMID: 25877923 Free PMC article. No abstract available.
Abstract
Two Krebs cycle genes, fumarate hydratase (FH) and succinate dehydrogenase (SDH), are mutated in a subset of human cancers, leading to accumulation of their substrates, fumarate and succinate, respectively. Here we demonstrate that fumarate and succinate are competitive inhibitors of multiple α-ketoglutarate (α-KG)-dependent dioxygenases, including histone demethylases, prolyl hydroxylases, collagen prolyl-4-hydroxylases, and the TET (ten-eleven translocation) family of 5-methlycytosine (5mC) hydroxylases. Knockdown of FH and SDH results in elevated intracellular levels of fumarate and succinate, respectively, which act as competitors of α-KG to broadly inhibit the activity of α-KG-dependent dioxygenases. In addition, ectopic expression of tumor-derived FH and SDH mutants inhibits histone demethylation and hydroxylation of 5mC. Our study suggests that tumor-derived FH and SDH mutations accumulate fumarate and succinate, leading to enzymatic inhibition of multiple α-KG-dependent dioxygenases and consequent alterations of genome-wide histone and DNA methylation. These epigenetic alterations associated with mutations of FH and SDH likely contribute to tumorigenesis.
Figures
Figure 1.
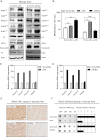
Fumarate and succinate inhibit the activity of α-KG-dependent KDMs in vitro. (A) Structural comparison among fumarate, succinate, α-KG, and 2-HG. (B) Fumarate and succinate inhibit the demethylase activity of C. elegans KDM7A (CeKDM7A). CeKDM7A activity toward monmethylated H3K9 was assayed in the presence or absence of 15 μM α-KG with increasing concentrations of either fumarate or succinate as indicated. The demethylated products were analyzed by mass spectrometry (MS), and mean activity values of duplicated assays, represented by percentage of remaining methylated peptides, are shown. (C) Fumarate and succinate inhibit the demethylase activity of human KDM4A (HsKDM4A) toward trimethylated H3K36, determined by mass spectrometry (MS) assay as in B. Error bars represent standard deviation (SD) for triplicate experiments. (D) Succinate is more potent to inhibit HsKDM4A than fumarate. The half maximal inhibitory concentration (IC50) of fumarate and succinate on HsKDM4A was determined by MS assay as in C. Error bars represent standard deviation (SD) for triplicate experiments. Additional MS results are shown in Supplemental Figure S1.
Figure 2.
Fumarate and succinate increase genome-wide histone methylations, accumulate HIF1α, and reduce endostatin in cultured cells. (A) Cell-permeable fumarate (2.5 mM) or succinate (5 mM) increases histone methylation and HIF1α and reduces endostatin in HEK293T cells (left) and HeLa cells (right), as determined by Western-blot. (B) Cell-permeable octyl-α-KG (5mM) diminishes the effect of FH or SDH suppression on increasing histone methylations, accumulating HIF1α, and decreasing endostatin in HEK293T cells with knockdown of FH (left panel), SDHA (middle panel), and SDHB (right panel), as determined by Western blot. See also Supplemental Figure S3.
Figure 3.
Knocking down FH or SDH reduces the TET-catalyzed 5hmC production in cultured cells. (A) HEK293T cells with stable knockdown of FH or SDHA/B were transiently transfected with plasmids expressing the indicated proteins. Thirty-six hours to 40 h after the transfection, cells were fixed and stained with antibodies specific to Flag to determine the expression of TET protein, with antibodies specific to 5hmC to determine the levels of 5hmC, and with antibodies specific to DAPI to view the cell nuclei. Bars, 251 μm. See also Supplemental Figure S4. (B_–_D) HEK293T cells were transiently transfected as described in A. Genomic DNAs were isolated from cells and immunoblotted with an antibody specific to 5hmC. Quantification of 5hmC was calculated from three independent assays. The expression of individual proteins was determined by immunoblotting as shown at the right. One representative quantification of the 5hmC level determined from the assays using 100 ng of genomic DNA is included. Error bars represent standard deviation (SD) for triplicate experiments.
Figure 4.
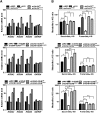
Knocking down Fh or Sdha inhibits multiple α-KG-dependent dioxygenases and regulates gene expression in vivo. (A) Mice with transient knockdown of Fh or Sdha in the liver were generated by using the hydrodynamic tail vein-based delivery technique (n = 3 per group). The Fh or Sdha knockdown efficiency and levels of histone methylation, HIF1α, and endostatin were determined by Western blot. (B) The ratios of succinate/α-KG and fumarate/α-KG in mouse livers after suppression of Fh or Sdha were determined by GC-MS. Error bars represent standard deviation (SD) for triplicate experiments. (**) P < 0.01 versus Scramble; (n.s.) not significant. See also Supplemental Figure S5. (C,D) The mRNA expression of Hoxa genes in mouse livers after suppression of Fh or Sdha was determined by quantitative RT–PCR. Error bars represent standard deviation (SD) for triplicate experiments. (E,F) The 5hmC levels in mouse livers after suppression of Fh or Sdha were determined by immunohistochemistry (E) and dot blot assay (F). Bars, 334 μm. Error bars represent standard deviation (SD) for triplicate experiments. See also Supplemental Figure S6.
Figure 5.
Ectopic expression of tumor-derived FH or SDH mutants inhibits α-KG-dependent dioxygenases in cultured cells. (A) HEK293T cells with stable FH or SDHA/B knockdown were transiently transfected with plasmids expressing the indicated proteins, and the enzyme activity of wild-type and mutant FH or SDH was measured as described in the Material and Methods. Error bars represent standard deviation (SD) for triplicate experiments. (**) P < 0.01 versus wild type; (##) P < 0.01 versus vector. (B) Overexpression of tumor-derived FH or SDHA/B mutants inhibits histone demethylation and hydroxylation of prolyl residues. Levels of histone methylation, HIF1α, and endostatin were determined by Western blot. (C) Overexpression of tumor-derived FH or SDHA/B mutants inhibits the TET-catalyzed 5mC oxidation. 5hmC levels were determined by dot blot. Error bars represent standard deviation (SD) for triplicate experiments.
Figure 6.
Tumor-derived FH or SDH mutants are not functional in fumarate or succinate metabolism. (A) HEK293T cells with stable FH or SDHA/B knockdown were transfected with the indicated plasmids. Quantitative RT–PCR was performed to determine mRNA expression of HOXA genes in these cells. Error bars represent standard deviation (SD) for triplicate experiments. (B) The ratios of succinate/α-KG and fumarate/α-KG were determined by GC-MS. Error bars represent standard deviation (SD) for triplicate experiments. (*) P < 0.05 versus Scramble;; (**) P < 0.01 versus Scramble; (n.s.) not significant. See also Supplemental Figure S9.
Figure 7.
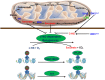
Fumarate and succinate can function as α-KG antagonists to broadly inhibit α-KG-dependent dioxygenases, including the JMJD family KDMs and the TET family of 5mC hydroxylases.
Similar articles
- Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma/pheochromocytoma and smooth muscle tumors.
Hoekstra AS, de Graaff MA, Briaire-de Bruijn IH, Ras C, Seifar RM, van Minderhout I, Cornelisse CJ, Hogendoorn PC, Breuning MH, Suijker J, Korpershoek E, Kunst HP, Frizzell N, Devilee P, Bayley JP, Bovée JV. Hoekstra AS, et al. Oncotarget. 2015 Nov 17;6(36):38777-88. doi: 10.18632/oncotarget.6091. Oncotarget. 2015. PMID: 26472283 Free PMC article. - Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases.
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Xu W, et al. Cancer Cell. 2011 Jan 18;19(1):17-30. doi: 10.1016/j.ccr.2010.12.014. Cancer Cell. 2011. PMID: 21251613 Free PMC article. - The role of metabolic enzymes in mesenchymal tumors and tumor syndromes: genetics, pathology, and molecular mechanisms.
Schaefer IM, Hornick JL, Bovée JVMG. Schaefer IM, et al. Lab Invest. 2018 Apr;98(4):414-426. doi: 10.1038/s41374-017-0003-6. Epub 2018 Jan 16. Lab Invest. 2018. PMID: 29339836 Review. - Exposure to high levels of fumarate and succinate leads to apoptotic cytotoxicity and altered global DNA methylation profiles in vitro.
Wentzel JF, Lewies A, Bronkhorst AJ, van Dyk E, du Plessis LH, Pretorius PJ. Wentzel JF, et al. Biochimie. 2017 Apr;135:28-34. doi: 10.1016/j.biochi.2017.01.004. Epub 2017 Jan 16. Biochimie. 2017. PMID: 28104508 - Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer.
King A, Selak MA, Gottlieb E. King A, et al. Oncogene. 2006 Aug 7;25(34):4675-82. doi: 10.1038/sj.onc.1209594. Oncogene. 2006. PMID: 16892081 Review.
Cited by
- Understanding metabolic regulation and its influence on cell physiology.
Metallo CM, Vander Heiden MG. Metallo CM, et al. Mol Cell. 2013 Feb 7;49(3):388-98. doi: 10.1016/j.molcel.2013.01.018. Mol Cell. 2013. PMID: 23395269 Free PMC article. Review. - Rethinking pheochromocytomas and paragangliomas from a genomic perspective.
Castro-Vega LJ, Lepoutre-Lussey C, Gimenez-Roqueplo AP, Favier J. Castro-Vega LJ, et al. Oncogene. 2016 Mar 3;35(9):1080-9. doi: 10.1038/onc.2015.172. Epub 2015 Jun 1. Oncogene. 2016. PMID: 26028031 Review. - Metabolic determinants of tumour initiation.
Brunner JS, Finley LWS. Brunner JS, et al. Nat Rev Endocrinol. 2023 Mar;19(3):134-150. doi: 10.1038/s41574-022-00773-5. Epub 2022 Nov 29. Nat Rev Endocrinol. 2023. PMID: 36446897 Free PMC article. Review. - Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism.
Aspuria PP, Lunt SY, Väremo L, Vergnes L, Gozo M, Beach JA, Salumbides B, Reue K, Wiedemeyer WR, Nielsen J, Karlan BY, Orsulic S. Aspuria PP, et al. Cancer Metab. 2014 Dec 15;2:21. doi: 10.1186/2049-3002-2-21. eCollection 2014. Cancer Metab. 2014. PMID: 25671108 Free PMC article. - Reciprocal regulation of amino acid import and epigenetic state through Lat1 and EZH2.
Dann SG, Ryskin M, Barsotti AM, Golas J, Shi C, Miranda M, Hosselet C, Lemon L, Lucas J, Karnoub M, Wang F, Myers JS, Garza SJ, Follettie MT, Geles KG, Klippel A, Rollins RA, Fantin VR. Dann SG, et al. EMBO J. 2015 Jul 2;34(13):1773-85. doi: 10.15252/embj.201488166. Epub 2015 May 15. EMBO J. 2015. PMID: 25979827 Free PMC article.
References
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. 2011. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20: 524–537 - PMC - PubMed
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, et al. 2011. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224: 306–308 - PubMed
- Bardella C, Pollard PJ, Tomlinson I 2011. SDH mutations in cancer. Biochim Biophys Acta 1807: 1432–1443 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous