Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP - PubMed (original) (raw)
Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP
Yi Liu-Chittenden et al. Genes Dev. 2012.
Abstract
The Drosophila TEAD ortholog Scalloped is required for Yki-mediated overgrowth but is largely dispensable for normal tissue growth, suggesting that its mammalian counterpart may be exploited for selective inhibition of oncogenic growth driven by YAP hyperactivation. Here we test this hypothesis genetically and pharmacologically. We show that a dominant-negative TEAD molecule does not perturb normal liver growth but potently suppresses hepatomegaly/tumorigenesis resulting from YAP overexpression or Neurofibromin 2 (NF2)/Merlin inactivation. We further identify verteporfin as a small molecule that inhibits TEAD-YAP association and YAP-induced liver overgrowth. These findings provide proof of principle that inhibiting TEAD-YAP interactions is a pharmacologically viable strategy against the YAP oncoprotein.
Figures
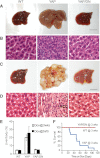
Figure 1.
TEAD2-DN suppressed hepatomegaly and tumorigenesis driven by YAP overexpression. (A,B) Whole amount (A) and hematoxylin/eosin (H&E) staining (B) of livers from wild-type (WT), YAP, and YAP/TEAD2-DN mice treated with 0.2 g/L Dox for 2 wk starting at 3 wk of age. Bar, 1 cm. (C,D) similar to A and B except that mice were treated with 1 g/L Dox for 8 wk starting at birth. (E) Quantification of liver-to-body weight ratio for animals analyzed in A and C. Values are mean ± SEM; n ≥ 3 for each data point. (F) Survival curves of wild-type, YAP, and YAP/TEAD2-DN mice subjected to 0.2 g/L Dox treatment starting at 3 wk of age.
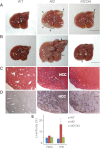
Figure 2.
TEAD2-DN suppressed _Nf2_-deficient phenotypes in the liver. (A) Livers from wild-type, Nf2, and Nf2 TEAD2-DN mice subjected to 2 g/L Dox treatment for 7 wk starting at gestation. Note the presence of thick hamartomas (arrowheads) at the edge of the Nf2 mutant liver. Only a remnant hamartoma (arrowhead) was visible in the Nf2 TEAD2-DN liver. Bar, 1cm. (B) Similar to A except that mice were subjected to Dox treatment for 1 yr starting at birth. Note the massive overgrowth and the presence of multiple HCC (arrows) in the Nf2 mutant liver and the near-normal appearance of the Nf2 TEAD2-DN liver. Note that bile duct hamartomas, which appeared first at the edge of the Nf2 mutant liver (shown in A), have invaded deep into the liver parenchyma by 1 yr of age, giving the Nf2 mutant liver an overall pale color. Only a remnant hamartoma (arrowhead) was visible in the Nf2 TEAD2-DN liver. (C,D) H&E (C) and CK (D) staining of liver sections from B. Note the presence of deep-penetrating CK-positive bile duct hamartoma and CK-negative HCC in the Nf2 mutant liver. Also note the greatly reduced bile duct hyperplasia and the absence of HCC in the Nf2 TEAD2-DN liver. (E) Quantification of liver-to-body weight ratio for animals analyzed in A and B. (7WG) Dox treatment for 7 wk starting at gestation; (1YB) Dox treatment for 1 yr starting at birth. n ≥ 6 for each data point. In both conditions, the overgrowth of Nf2 mutant livers was significantly suppressed in Nf2 TEAD2-DN livers (P = 1 × 10−5 and 1 × 10−7, respectively).
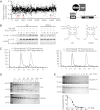
Figure 3.
Identification of VP and related porphyrin compounds as inhibitors of TEAD–YAP interactions. (A) Plot showing distribution of luciferase activity of HEK293 cells expressing Gal4-TEAD4/YAP/UAS-Luc and treated with individual Hopkins Library compounds at 10 μM. All three porphyrin derivatives from the library (PPIX, HP, and VP) were scored as hit compounds as circled. A schematic drawing of the luciferase assay is also shown. (B) Inhibition of TEAD–YAP interaction by VP and PPIX. HEK293 cells expressing Gal4-TEAD4 and HA-YAP were incubated with the indicated concentrations of each chemical, and the presence of Gal4-TEAD in the HA-YAP immunoprecipitates was probed. The chemical structures of VP and PPIX are also shown. (C) VP binds to purified YAP in vitro. Purified YAP (5 μM) (left) or TEAD2 (5 μM) (right) was incubated with VP (15 μM) for 15 min at room temperature. The mixture was then fractioned through a size exclusion column, and the VP fluorescence was measured in each fraction. Note that an appreciable VP fluorescence coeluted with the YAP protein peak (fraction 45, left) but not with the TEAD2 protein peak (fraction 54, right). (D) Effect of VP on trypsin cleavage of YAP or TEAD2. Purified YAP or TEAD2 (5 μM) was preincubated with DMSO (D) or 20 μM VP (V) for 30 min at room temperature prior to trypsin digestion for the indicated time. The cleavage products were analyzed by Coomasie brilliant blue staining after SDS-PAGE. (E) Effect of different concentrations of VP on trypsin cleavage of YAP. YAP (5 μM) was preincubated with various concentrations of VP for 30 min at room temperature, followed by incubation with (+) or without (−) trypsin (0.1 μg/mL) for 1 h on ice. The graph shows quantification of uncleaved versus input YAP at various VP concentrations.
Figure 4.
VP suppressed liver overgrowth caused by YAP overexpression or inactivation of Nf2. (A) Experimental design. Three-week-old YAP transgenic mice were fed 20 mg/L Dox for 8 d. VP suspension (100 mg/kg) was administered by intraperitoneal injection once every other day during the 8-d period. (B) Livers from control (Dox+DMSO; left picture) and VP-treated (Dox+VP; right picture) YAP transgenic mice (left) and quantification of liver-to-body weight ratio (right) at the end of the 8-d period. The VP-treated mice had significantly smaller livers than control livers (7.3% vs. 8.9%, n = 5 in each group, P < 0.001, _t_-test). (C) Phospho-H3 (PH3) staining of liver sections (left) and quantification of PH3-positive cells (right) of liver sections from B. (D) Experimental design. Timed pregnant Nf2flox2/flox2 mothers (after mating with Alb-Cre; Nf2flox2/flox2 males) received intraperitoneal injection of VP (100 mg/kg) or DMSO every other day starting at 9 d after detection of vaginal plugs (corresponding to E9.5 for embryos carried by the pregnant mothers). Newborn pups (corresponding to E18.5) were analyzed for liver phenotypes. (E) CK staining of liver sections from E18.5 Alb-Cre; Nf2flox2/flox2 pups that had been subjected to control (DMSO) (left) or VP (right) treatment from E9.5 to E18.5. The graph shows quantification of CK-positive BECs.
Comment in
- Quit your YAPing: a new target for cancer therapy.
Stanger BZ. Stanger BZ. Genes Dev. 2012 Jun 15;26(12):1263-7. doi: 10.1101/gad.196501.112. Genes Dev. 2012. PMID: 22713867 Free PMC article.
Similar articles
- A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis.
Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, Guan KL. Moroishi T, et al. Genes Dev. 2015 Jun 15;29(12):1271-84. doi: 10.1101/gad.262816.115. Genes Dev. 2015. PMID: 26109050 Free PMC article. - TEAD mediates YAP-dependent gene induction and growth control.
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. Zhao B, et al. Genes Dev. 2008 Jul 15;22(14):1962-71. doi: 10.1101/gad.1664408. Epub 2008 Jun 25. Genes Dev. 2008. PMID: 18579750 Free PMC article. - The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity.
Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. Shimomura T, et al. Biochem Biophys Res Commun. 2014 Jan 17;443(3):917-23. doi: 10.1016/j.bbrc.2013.12.100. Epub 2013 Dec 28. Biochem Biophys Res Commun. 2014. PMID: 24380865 - WWC1 and NF2 Prevent the Development of Intrahepatic Cholangiocarcinoma by Regulating YAP/TAZ Activity through LATS in Mice.
Park J, Kim JS, Nahm JH, Kim SK, Lee DH, Lim DS. Park J, et al. Mol Cells. 2020 May 31;43(5):491-499. doi: 10.14348/molcells.2020.0093. Mol Cells. 2020. PMID: 32451369 Free PMC article. - The TEAD Family and Its Oncogenic Role in Promoting Tumorigenesis.
Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. Zhou Y, et al. Int J Mol Sci. 2016 Jan 21;17(1):138. doi: 10.3390/ijms17010138. Int J Mol Sci. 2016. PMID: 26805820 Free PMC article. Review.
Cited by
- In vivo genome-wide CRISPR screen reveals breast cancer vulnerabilities and synergistic mTOR/Hippo targeted combination therapy.
Dai M, Yan G, Wang N, Daliah G, Edick AM, Poulet S, Boudreault J, Ali S, Burgos SA, Lebrun JJ. Dai M, et al. Nat Commun. 2021 May 24;12(1):3055. doi: 10.1038/s41467-021-23316-4. Nat Commun. 2021. PMID: 34031411 Free PMC article. - The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression.
Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. Koontz LM, et al. Dev Cell. 2013 May 28;25(4):388-401. doi: 10.1016/j.devcel.2013.04.021. Dev Cell. 2013. PMID: 23725764 Free PMC article. - Hippo Signaling Mediates TGFβ-Dependent Transcriptional Inputs in Cardiac Cushion Mesenchymal Cells to Regulate Extracellular Matrix Remodeling.
Chakrabarti M, Chattha A, Nair A, Jiao K, Potts JD, Wang L, Branch S, Harrelson S, Khan S, Azhar M. Chakrabarti M, et al. J Cardiovasc Dev Dis. 2023 Dec 4;10(12):483. doi: 10.3390/jcdd10120483. J Cardiovasc Dev Dis. 2023. PMID: 38132651 Free PMC article. - Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma.
Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang L, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K. Tschaharganeh DF, et al. Gastroenterology. 2013 Jun;144(7):1530-1542.e12. doi: 10.1053/j.gastro.2013.02.009. Epub 2013 Feb 16. Gastroenterology. 2013. PMID: 23419361 Free PMC article. - Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice.
Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, Bai Y, Li W, Willerson JT, Martin JF. Morikawa Y, et al. Sci Signal. 2015 May 5;8(375):ra41. doi: 10.1126/scisignal.2005781. Sci Signal. 2015. PMID: 25943351 Free PMC article.
References
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060 - PubMed
- Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ Jr, Liu JO 2007. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol 2: 263–270 - PubMed
- Chow L, Berube J, Fromont A, Bell JB 2004. Ability of scalloped deletion constructs to rescue sd mutant wing phenotypes in Drosophila melanogaster. Genome 47: 849–859 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA122814/CA/NCI NIH HHS/United States
- R01EY015708/EY/NEI NIH HHS/United States
- R01 AR060636/AR/NIAMS NIH HHS/United States
- R01DK081417/DK/NIDDK NIH HHS/United States
- R01AR060636/AR/NIAMS NIH HHS/United States
- R01 EY015708/EY/NEI NIH HHS/United States
- R01 DK081417/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous