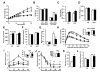The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity - PubMed (original) (raw)
. 2012 Jun 6;15(6):838-47.
doi: 10.1016/j.cmet.2012.04.022.
Riekelt H Houtkooper, Eija Pirinen, Dou Y Youn, Maaike H Oosterveer, Yana Cen, Pablo J Fernandez-Marcos, Hiroyasu Yamamoto, Pénélope A Andreux, Philippe Cettour-Rose, Karl Gademann, Chris Rinsch, Kristina Schoonjans, Anthony A Sauve, Johan Auwerx
Affiliations
- PMID: 22682224
- PMCID: PMC3616313
- DOI: 10.1016/j.cmet.2012.04.022
The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity
Carles Cantó et al. Cell Metab. 2012.
Abstract
As NAD(+) is a rate-limiting cosubstrate for the sirtuin enzymes, its modulation is emerging as a valuable tool to regulate sirtuin function and, consequently, oxidative metabolism. In line with this premise, decreased activity of PARP-1 or CD38-both NAD(+) consumers-increases NAD(+) bioavailability, resulting in SIRT1 activation and protection against metabolic disease. Here we evaluated whether similar effects could be achieved by increasing the supply of nicotinamide riboside (NR), a recently described natural NAD(+) precursor with the ability to increase NAD(+) levels, Sir2-dependent gene silencing, and replicative life span in yeast. We show that NR supplementation in mammalian cells and mouse tissues increases NAD(+) levels and activates SIRT1 and SIRT3, culminating in enhanced oxidative metabolism and protection against high-fat diet-induced metabolic abnormalities. Consequently, our results indicate that the natural vitamin NR could be used as a nutritional supplement to ameliorate metabolic and age-related disorders characterized by defective mitochondrial function.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1. Nicotinamide Riboside supplementation increases NAD+ content and sirtuin activity in cultured mammalian cells
(A) C2C12 myotubes, Hepa1.6 and HEK293T cells were treated with nicotinamide riboside (NR) for 24 hrs and acidic extracts were obtained to measure total NAD+ intracellular content. (B) GPR109A-expressing Chem-4 cells were loaded with 3 μM Fura-2 acetoxymethyl ester derivative (Fura-2/AM) for 30 min at 37 °C. Then, cells were washed with Hank’s balanced salt solution and calcium flux in response to nicotinic acid (NA; as positive control), NR and nicotinamide mononucleotide (NMN) at the concentrations indicated was determined as indicated in methods. (C) C2C12 myotubes, Hepa1.6 and HEK293T cells were treated with either PBS (as Vehicle) or 0.5 mM of NR, NMN or NA for 24 hrs. Then total NAD+ intracellular content was determined as in (A). (D) C57Bl/6J mice were fed with chow containing vehicle (water) or either NR, NMN or NA at 400 mg/kg/day (n=8 mice per group). After one week, NAD+ content was determined in liver and quadriceps muscle. (E) HEK293T cells were treated with NR (0.5 mM, black bars) or vehicle (white bars) for 4 hrs. Then, cells were harvested and mitochondria were isolated for NAD+ measurement. (F) C57Bl/6J mice were fed with chow containing vehicle (water) or NR at 400 mg/kg/day (n=8 mice per group). After one week, mitochondria were isolated from their livers to measure NAD+ content. (G) HEK293T cells were treated with either PBS (as Vehicle) or 0.5 mM of NR for 24 hrs. Then mRNA and protein was extracted to measure Nampt levels by RT-qPCR and western blot, respectively. (H) HEK293T cells were treated with either PBS (as Vehicle) or 0.5 mM of NR for 24 hrs. Then protein homogenates were obtained to test global PARylation and PARP-1 levels. Throughout the figure, all values are presented as mean +/− SD. * indicates statistical significant difference vs. respective vehicle group at P < 0.05. Unless otherwise stated, the vehicle groups are represented by white bars, and NR groups are represented by black bars.
Figure 2. Nicotinamide Riboside supplementation increases sirtuin activity in cultured mammalian cells
(A) HEK293T cells were transfected with a pool of either scramble siRNAs or SIRT1 siRNAs. After 24 hrs, cells were treated with vehicle (PBS) or NR at the concentrations indicated, and, after an additional 24 hrs, total protein extracts were obtained. FOXO1 acetylation was tested after FOXO1 immunoprecipitation (IP) from 500 μg of protein, while tubulin and SIRT1 levels were evaluated in the supernatant of the IP. (B) HEK293T cells were transfected with a pool of either scramble siRNAs, FOXO1 siRNAs or SIRT1 siRNAs. After 24 hrs, cells were treated with NR (0.5 mM; black bars) or vehicle (PBS; white bars) for additional 24 hrs. Then total mRNA was extracted and the mRNA expression levels of the markers indicated was evaluated by qRT-PCR. (C) HEK293T cells were transfected with a pool of either scramble siRNAs, FOXO1 siRNAs or SIRT1 siRNAs. After 24 hrs, cells were treated with NR (0.5 mM; black bars) or vehicle (PBS; white bars) for additional 24 hrs. Then acidic extracts were obtained to measure intracellular NAD+ levels. (D-E) HEK293T cells were treated with NR (0.5 mM) or vehicle (PBS) for 24 hrs and total protein extracts were obtained to measure (D) Ndufa9 or (E) SOD2 acetylation after IP. The extracts were also used to measure SOD2 activity (E, bottom panel). (F-G). SIRT3+/+ and SIRT3−/− mouse embryonic fibroblasts (MEFs) were treated with NR (0.5 mM) or vehicle (PBS) for 24 hrs and either (F) total extracts to test SOD2 acetylation were obtained or (G) acidic extracts were used to measure intracellular NAD+ content. Throughout the figure, all values are presented as mean +/− SD. * indicates statistical significant difference vs. respective vehicle group at P < 0.05. Unless otherwise stated, the vehicle groups are represented by white bars, and NR groups are represented by black bars. This figure is complemented by Fig.S1
Figure 3. NR supplementation prevents diet-induced obesity by enhancing energy expenditure and reduces cholesterol levels
10-week-old C57Bl/6J mice were fed with either chow (CD) or high fat diet (HFD) mixed with either water (as vehicle) or NR (400 mg/kg/day) (n=10 mice per group). (A) Body weight evolution was monitored during 12 weeks. (B) Body composition was evaluated after 8 weeks of diet through Echo-MRI. (C-E) Food intake, activity and VO2 were evaluated using indirect calorimetry. (F-G) Blood glucose and insulin levels were measured in animals fed with their respective diets for 16 weeks after a 6 hr fast. (H-I) After 10 weeks on their respective diets (CD = squares; HFD = circles) an intraperitoneal glucose tolerance test was performed in mice that were fasted overnight. At the indicated times blood samples were obtained to evaluate either (H) glucose or (I) insulin levels. Areas under the curve are shown at the top-right of the respective panels. (J) Insulin tolerance tests were performed on either CD or CD-NR mice (4 weeks of treatment). At the indicated times, blood samples were obtained to evaluate blood glucose levels. The area above the curve is shown at the top-right of the panel. (K) Hyperinsulinemic-euglycemic clamps were performed on either CD or CD-NR mice (4 weeks of treatment). Glucose infusion rates (GIR) were calculated after the test. (L) Serum levels of total cholesterol were measured in animals fed with their respective diets for 16 weeks, after a 6 hr fast. Throughout the figure, white represent the vehicle group and black represent the NR-supplemented mice. All values are presented as mean +/− SD. * indicates statistical significant difference vs. respective vehicle treated group. This figure is complemented by Fig.S2
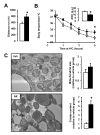
Figure 4. NR enhances skeletal muscle and BAT oxidative function
10-week-old C57Bl/6J mice were fed a high fat diet (HFD) mixed with either water (as vehicle; white bars and circles) or NR (400 mg/kg/day; black bars and circles) (n=10 mice per group). (A) An endurance exercise test was performed using a treadmill in mice fed with either HFD or HFD-NR for 12 weeks. (B) A cold-test was performed in mice fed with either HFD or HFD-NR for 9 weeks. The area over the curve (AOC) is shown on the top right of the graph. (D) Electron microscopy of the BAT was used to analyze mitochondrial content and morphology. The size and cristae content of mitochondria was quantified as specified in methods. Throughout the figure, all values are shown as mean +/− SD. * indicates statistical significant difference vs. vehicle supplemented group at P< 0.05. This figure is complemented by Fig.S3
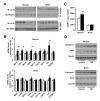
Figure 5. Chronic NR supplementation increases plasma and intracellular NAD+ content in a tissue-specific manner
Tissues from C57Bl/6J mice were collected after 16 weeks of HFD supplemented with either water (as vehicle; white bars) or NR (400 mg/kg/day; black bars). (A) NAD+ levels were measured in acidic extracts obtained from different tissues. (B) NADH and NAM levels were measured in gastrocnemius muscle. (C) Quadriceps muscle protein homogenates were obtained to test global PARylation, PARP-1 and Nampt protein levels. (D) Total mRNA was isolated from quadriceps muscles and the mRNA levels of the markers indicated were measured by RT-qPCR. Throughout the figure, all values are expressed as mean +/− SD. * indicates statistical significant difference vs. respective vehicle treated group.
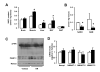
Figure 6. NR stimulates sirtuin activity in vivo and enhances mitochondrial gene expression
Tissues from C57Bl/6J mice were collected after 16 weeks of HFD supplemented with either water (as vehicle; white bars) or NR (400 mg/kg/day; black bars). (A) Total protein extracts were obtained from quadriceps muscle and brain indicated to evaluate the acetylation levels of FOXO1 and SOD2 through immunoprecipitation assays, using 1 and 0.5 mg of protein, respectively. (B) Total mRNA from quadriceps muscle and brain was extracted to measure the abundance of the markers indicated by RT-qPCR. (C) Mitochondrial DNA content was measured in DNA extracted from quadriceps muscle and brain. The results are expressed a mitochondrial copy number relative to genomic DNA (D) The abundance of mitochondrial marker proteins in 20 μg of protein from total quadriceps muscle and brain lysates. Throughout the figure, all values are shown as mean +/− SD. * indicates statistical significant difference vs. vehicle supplemented group at P< 0.05. This figure is complemented by Fig.S4
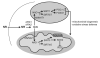
Figure 7. Schematic representation of the different actions of NR in metabolic homeostasis
The scheme summarizes the hypothesis by which NR supplementation would increase NAD+ content in key metabolic tissues, leading to SIRT1 and SIRT3 activation and the deacetylation and modulation of the activity of key metabolic regulators. This model does not rule out the participation of additional mechanisms of action for NR to achieve its beneficial effects. Abbreviations can be found in the text and enzymes are indicated in italics.
Similar articles
- Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis.
Pham TX, Bae M, Kim MB, Lee Y, Hu S, Kang H, Park YK, Lee JY. Pham TX, et al. Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2451-2463. doi: 10.1016/j.bbadis.2019.06.009. Epub 2019 Jun 11. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31195117 Free PMC article. - Nicotinamide Riboside Supplementation to Suckling Male Mice Improves Lipid and Energy Metabolism in Skeletal Muscle and Liver in Adulthood.
Serrano A, Palou A, Bonet ML, Ribot J. Serrano A, et al. Nutrients. 2022 May 28;14(11):2259. doi: 10.3390/nu14112259. Nutrients. 2022. PMID: 35684059 Free PMC article. - Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3.
Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Khan NA, et al. EMBO Mol Med. 2014 Jun;6(6):721-31. doi: 10.1002/emmm.201403943. EMBO Mol Med. 2014. PMID: 24711540 Free PMC article. - Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection.
Chi Y, Sauve AA. Chi Y, et al. Curr Opin Clin Nutr Metab Care. 2013 Nov;16(6):657-61. doi: 10.1097/MCO.0b013e32836510c0. Curr Opin Clin Nutr Metab Care. 2013. PMID: 24071780 Review. - Interplay between NADH oxidation by complex I, glutathione redox state and sirtuin-3, and its role in the development of insulin resistance.
Cortés-Rojo C, Vargas-Vargas MA, Olmos-Orizaba BE, Rodríguez-Orozco AR, Calderón-Cortés E. Cortés-Rojo C, et al. Biochim Biophys Acta Mol Basis Dis. 2020 Aug 1;1866(8):165801. doi: 10.1016/j.bbadis.2020.165801. Epub 2020 Apr 16. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32305451 Review.
Cited by
- Novel directions for diabetes mellitus drug discovery.
Maiese K, Chong ZZ, Shang YC, Wang S. Maiese K, et al. Expert Opin Drug Discov. 2013 Jan;8(1):35-48. doi: 10.1517/17460441.2013.736485. Epub 2012 Oct 24. Expert Opin Drug Discov. 2013. PMID: 23092114 Free PMC article. Review. - The mitochondrial unfolded protein response (UPRmt) protects against osteoarthritis.
Zhou Z, Lu J, Yang M, Cai J, Fu Q, Ma J, Zhu L. Zhou Z, et al. Exp Mol Med. 2022 Nov;54(11):1979-1990. doi: 10.1038/s12276-022-00885-y. Epub 2022 Nov 15. Exp Mol Med. 2022. PMID: 36380018 Free PMC article. - Protective effects of sirtuins in cardiovascular diseases: from bench to bedside.
Winnik S, Auwerx J, Sinclair DA, Matter CM. Winnik S, et al. Eur Heart J. 2015 Dec 21;36(48):3404-12. doi: 10.1093/eurheartj/ehv290. Epub 2015 Jun 25. Eur Heart J. 2015. PMID: 26112889 Free PMC article. Review. - NADP modulates RNA m6A methylation and adipogenesis via enhancing FTO activity.
Wang L, Song C, Wang N, Li S, Liu Q, Sun Z, Wang K, Yu SC, Yang Q. Wang L, et al. Nat Chem Biol. 2020 Dec;16(12):1394-1402. doi: 10.1038/s41589-020-0601-2. Epub 2020 Jul 27. Nat Chem Biol. 2020. PMID: 32719557 - Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake.
Merz KE, Thurmond DC. Merz KE, et al. Compr Physiol. 2020 Jul 8;10(3):785-809. doi: 10.1002/cphy.c190029. Compr Physiol. 2020. PMID: 32940941 Free PMC article. Review.
References
- Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. - PubMed
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous