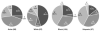Understanding vaginal microbiome complexity from an ecological perspective - PubMed (original) (raw)
Review
Understanding vaginal microbiome complexity from an ecological perspective
Roxana J Hickey et al. Transl Res. 2012 Oct.
Abstract
The various microbiota normally associated with the human body have an important influence on human development, physiology, immunity, and nutrition. This is certainly true for the vagina wherein communities of mutualistic bacteria constitute the first line of defense for the host by excluding invasive, nonindigenous organisms that may cause disease. In recent years much has been learned about the bacterial species composition of these communities and how they differ between individuals of different ages and ethnicities. A deeper understanding of their origins and the interrelationships of constituent species is needed to understand how and why they change over time or in response to changes in the host environment. Moreover, there are few unifying theories to explain the ecological dynamics of vaginal ecosystems as they respond to disturbances caused by menses and human activities such as intercourse, douching, and other habits and practices. This fundamental knowledge is needed to diagnose and assess risk to disease. Here we summarize what is known about the species composition, structure, and function of bacterial communities in the human vagina and the applicability of ecological models of community structure and function to understanding the dynamics of this and other ecosystems that comprise the human microbiome.
Copyright © 2012 Mosby, Inc. All rights reserved.
Figures
Figure 1
Representation of vaginal bacterial community groups within four ethnic groups of women. The number of women from each ethnic group is in parentheses. The roman numerals indicate the five common vaginal bacterial community groups described by Ravel et al. Community groups I, II, III and V are predominated by Lactobacillus crispatus, L. gasseri, L. iners and L. jensenii, respectively, while community group IV contains a diverse assemblage of facultative and strictly anaerobic bacteria. Percent values are the percentages of women in each ethnic group whose vaginal bacterial community clustered with a particular community group. (reproduced from data in reference 4)
Figure 2
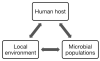
The vaginal ecosystem and bacterial communities therein are strongly influenced by characteristics of the host, local environment, and constituent populations.
Figure 3
The resistance or ‘quasi-stability’ of a community reflects its capacity to resist change in structure in response to a disturbance event. Ecosystem disturbances can occur at varying intensities and frequencies or durations, indicated here on the y-axis (magnitude or intensity) and x-axis (frequency or duration), respectively. Panels (A) and (B) represent two communities with different levels of resistance. The lighter portion in the bottom left-hand portion of each space represents an ecosystem’s quasi-stable state in which changes may occur to the community structure without pushing it into a ‘disturbed’ state. The darker portion in the upper right represents the disturbed ecosystem. Circles surrounded by dashed lines with an “_i_” inside represent various initial states of an ecosystem, and circles with solid lines and “_f_” inside represent the final state following a disturbance event. Some disturbances may push the ecosystem to another point within its quasi-stable space (e.g., f1 in [_A_]; f1 and f2 in [_B_]) whereas some disturbances may be great enough to push the community into a ‘disturbed’ state (e.g., f2 and f3 in [_A_]; f3 in [_B_]). Communities that differ in species composition are likely to have different degrees of resistance. In our example, communities A and B experience the same disturbances, but in (A) disturbance events 2 and 3 push the community into a disturbed state whereas in (B) only disturbance event 3 is strong enough to disturb the ecosystem from its quasi-stable state.
Figure 4
Resilience is the ability of a community to return to a quasi-stable state following a disturbance event. Different communities, particularly if they differ in species composition, are presumed to possess different degrees of resilience. The x-axis and y-axis are the same as in Figure 3. Panels (A) and (B) represent two communities with different levels of resilience. Circles surrounded by dashed lines with an “_i_” inside represent various initial states of an ecosystem, dashed circles with “_d_” represent intermediate disturbed states, and circles with solid lines and “_f_” inside represent the final state following the disturbance. Dashed arrows indicate disturbance events (these are the same location, direction and magnitude in [_A_] and [_B_]), and the solid arrows indicate the community rebounding toward its quasi-stable state. The thickness of the solid line represents the relative degree of resilience. In this case, the resilience of community A is sufficient to restore quasi-stability in disturbance event 1 but not 2, whereas the resilience of community B is sufficient to recover from both disturbance events.
Figure 5
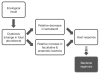
Possible models for the pathogenesis of bacterial vaginosis (BV). Following an ecological insult or disturbance, dysbiosis may result when there is a change in the total abundance of microorganisms. This could result in a relative decrease in lactobacilli or a relative increase in facultative and anaerobic bacteria. Both scenarios may elicit a host response that eventually results in BV. This schematic is modified from one presented by Srinivasan and Fredricks wherein the two models were referred to as the ‘Lactobacillus depletion model’ and ‘primary pathogen model.’
Figure 6
A phylogenetic tree showing the relationship of selected phylotypes from vaginal communities of healthy Caucasian and black women (marked by triangles), type strains from the RDP database (unmarked) and three BV-associated bacteria (BVAB) (marked with arrows; sequences deposited by Fredricks et al.). The phylogenetic tree was constructed using a neighbor-joining algorithm, with Mycoplasma spp. serving as the out-group. Bootstrap values (from 500 replicates) greater than 50% are shown at the branch points, and the bar indicates 10% sequence divergence.
Similar articles
- Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English. - Ecological dynamics of the vaginal microbiome in relation to health and disease.
Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Greenbaum S, et al. Am J Obstet Gynecol. 2019 Apr;220(4):324-335. doi: 10.1016/j.ajog.2018.11.1089. Epub 2018 Nov 14. Am J Obstet Gynecol. 2019. PMID: 30447213 Review. - Amylases in the Human Vagina.
Nunn KL, Clair GC, Adkins JN, Engbrecht K, Fillmore T, Forney LJ. Nunn KL, et al. mSphere. 2020 Dec 9;5(6):e00943-20. doi: 10.1128/mSphere.00943-20. mSphere. 2020. PMID: 33298571 Free PMC article. - Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis.
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. Ling Z, et al. BMC Genomics. 2010 Sep 7;11:488. doi: 10.1186/1471-2164-11-488. BMC Genomics. 2010. PMID: 20819230 Free PMC article. - Vaginal microbiome: rethinking health and disease.
Ma B, Forney LJ, Ravel J. Ma B, et al. Annu Rev Microbiol. 2012;66:371-89. doi: 10.1146/annurev-micro-092611-150157. Epub 2012 Jun 28. Annu Rev Microbiol. 2012. PMID: 22746335 Free PMC article. Review.
Cited by
- Changes in the Vaginal Microbiome and Associated Toxicities Following Radiation Therapy for Gynecologic Cancers.
Tsementzi D, Meador R, Eng T, Patel P, Shelton J, Arluck J, Scott I, Dolan M, Khanna N, Konstantinidis KT, Bruner DW. Tsementzi D, et al. Front Cell Infect Microbiol. 2021 Oct 27;11:680038. doi: 10.3389/fcimb.2021.680038. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34778097 Free PMC article. - The interplay between human papillomavirus and vaginal microbiota in cervical cancer development.
Sharifian K, Shoja Z, Jalilvand S. Sharifian K, et al. Virol J. 2023 Apr 19;20(1):73. doi: 10.1186/s12985-023-02037-8. Virol J. 2023. PMID: 37076931 Free PMC article. Review. - Ecology meets reproductive medicine in HIV prevention: the case for geography-informed approaches for bacterial vaginosis in Africa.
Passmore JS, Ngcapu S, Gitome S, Kullin BR, Welp K, Martin DP, Potloane D, Manhanzva MT, Obimbo MM, Gill K, Fevre ML, Happel AU, Jaspan HB, Kasaro M, Bukusi EA. Passmore JS, et al. Front Reprod Health. 2024 Nov 27;6:1431306. doi: 10.3389/frph.2024.1431306. eCollection 2024. Front Reprod Health. 2024. PMID: 39665036 Free PMC article. Review. - A Study of the Vaginal Microbiome in Healthy Canadian Women Utilizing cpn60-Based Molecular Profiling Reveals Distinct Gardnerella Subgroup Community State Types.
Albert AY, Chaban B, Wagner EC, Schellenberg JJ, Links MG, van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, Money D; VOGUE Research Group. Albert AY, et al. PLoS One. 2015 Aug 12;10(8):e0135620. doi: 10.1371/journal.pone.0135620. eCollection 2015. PLoS One. 2015. PMID: 26266808 Free PMC article. - The role of Lactobacillus species in the control of Candida via biotrophic interactions.
Zangl I, Pap IJ, Aspöck C, Schüller C. Zangl I, et al. Microb Cell. 2019 Nov 25;7(1):1-14. doi: 10.15698/mic2020.01.702. Microb Cell. 2019. PMID: 31921929 Free PMC article. Review.
References
- Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol. 2010;203:1.e1–1.e5. - PubMed
- Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME Journal. 2007;1:121–133. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources