Clinical benefit of response in chronic graft-versus-host disease - PubMed (original) (raw)
Multicenter Study
. 2012 Oct;18(10):1517-24.
doi: 10.1016/j.bbmt.2012.05.016. Epub 2012 Jun 6.
Paul J Martin, Xiaoyu Chai, Madan Jagasia, Jeanne Palmer, Joseph Pidala, Corey Cutler, Steven Z Pavletic, Mukta Arora, David Jacobsohn, Paul A Carpenter, Mary E D Flowers, Nandita Khera, Georgia B Vogelsang, Daniel Weisdorf, Barry E Storer, Stephanie J Lee; Chronic GVHD Consortium
Affiliations
- PMID: 22683612
- PMCID: PMC3443259
- DOI: 10.1016/j.bbmt.2012.05.016
Multicenter Study
Clinical benefit of response in chronic graft-versus-host disease
Yoshihiro Inamoto et al. Biol Blood Marrow Transplant. 2012 Oct.
Abstract
To determine whether changes in objective response measures proposed by the National Institutes of Health correlate with clinical benefit, such as symptom burden, quality of life, and survival outcomes, we analyzed data from a multicenter prospective cohort of 283 patients with chronic graft-versus-host disease requiring systemic treatment. The median follow-up time of survivors was 25.1 months (range, 5.4-47.7 months) after enrollment. Symptom measures included the Lee symptom scale and 10-point patient-reported symptoms. Quality-of-life measures included the Short Form-36, Functional Assessment of Cancer Therapy-Bone Marrow Transplantation, and Human Activities Profile. Overall and organ-specific responses were calculated by comparing manifestations at the 6-month visit and those at the enrollment visit using a provisional algorithm. Complete or partial responses were considered "response," and stable or progressive disease was considered "no response." Overall response rate at 6 months was 32%. Organ-specific response rates were 45% for skin, 23% for eyes, 32% for mouth, and 51% for gastrointestinal tract. Response at 6 months, as calculated according to the provisional response algorithm, was correlated with changes in symptom burden in patients with newly diagnosed chronic graft-versus-host disease, but not with changes in quality of life or survival outcomes. Modification of the algorithm or validation of other more meaningful clinical endpoints is warranted for future clinical trials of treatment for chronic graft-versus-host disease.
Copyright © 2012 American Society for Blood and Marrow Transplantation. All rights reserved.
Figures
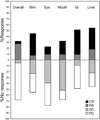
Figure 1
Calculated response for overall and individual organs at 6 months after enrollment, according to the provisional algorithm. GI, gastrointestinal tract; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 2
Survival outcomes according to overall response at 6 months. (A) Overall survival. (B) Nonrelapse mortality. P values are derived from the adjusted Cox model.
Similar articles
- Poor agreement between clinician response ratings and calculated response measures in patients with chronic graft-versus-host disease.
Palmer JM, Lee SJ, Chai X, Storer BE, Flowers ME, Schultz KR, Inamoto Y, Cutler C, Pidala J, Arora M, Jacobsohn DA, Carpenter PA, Pavletic SZ, Martin PJ. Palmer JM, et al. Biol Blood Marrow Transplant. 2012 Nov;18(11):1649-55. doi: 10.1016/j.bbmt.2012.05.005. Epub 2012 Jun 9. Biol Blood Marrow Transplant. 2012. PMID: 22691695 Free PMC article. - Content Validity of the Lee Chronic Graft-versus-Host Disease Symptom Scale as Assessed by Cognitive Interviews.
Merkel EC, Mitchell SA, Lee SJ. Merkel EC, et al. Biol Blood Marrow Transplant. 2016 Apr;22(4):752-758. doi: 10.1016/j.bbmt.2015.12.026. Epub 2016 Jan 2. Biol Blood Marrow Transplant. 2016. PMID: 26751003 Free PMC article. - Hand grip strength and 2-minute walk test in chronic graft-versus-host disease assessment: analysis from the Chronic GVHD Consortium.
Pidala J, Chai X, Martin P, Inamoto Y, Cutler C, Palmer J, Weisdorf D, Pavletic S, Arora M, Jagasia M, Jacobsohn D, Lee SJ. Pidala J, et al. Biol Blood Marrow Transplant. 2013 Jun;19(6):967-72. doi: 10.1016/j.bbmt.2013.03.014. Epub 2013 Mar 27. Biol Blood Marrow Transplant. 2013. PMID: 23542686 Free PMC article. - [Histopathology of graft-versus-host disease].
Länger F, Puls F, Buchholz S, Loddenkemper C, Ganser A, Kreipe H. Länger F, et al. Pathologe. 2011 Mar;32(2):144-51. doi: 10.1007/s00292-010-1408-9. Pathologe. 2011. PMID: 21279360 Review. German. - Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation.
Gratwohl A, Hermans J, Apperley J, Arcese W, Bacigalupo A, Bandini G, di Bartolomeo P, Boogaerts M, Bosi A, Carreras E, et al. Gratwohl A, et al. Blood. 1995 Jul 15;86(2):813-8. Blood. 1995. PMID: 7606012 Review.
Cited by
- Chronic GVHD: review advances in prevention, novel endpoints, and targeted strategies.
Amanam I, Otoukesh S, Al Malki MM, Salhotra A. Amanam I, et al. Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):164-170. doi: 10.1182/hematology.2023000427. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066845 Free PMC article. - Patient-reported treatment response in chronic graft-_versus_-host disease.
Greinix HT. Greinix HT. Haematologica. 2024 Jan 1;109(1):11-12. doi: 10.3324/haematol.2023.283524. Haematologica. 2024. PMID: 37470158 Free PMC article. No abstract available. - Patient-reported treatment response in chronic graft-_versus_-host disease.
Im A, Pusic I, Onstad L, Kitko CL, Hamilton BK, Alousi AM, Flowers ME, Sarantopoulos S, Carpenter P, White J, Arai S, El Jurdi N, Chen G, Cutler C, Lee S, Pidala J. Im A, et al. Haematologica. 2024 Jan 1;109(1):143-150. doi: 10.3324/haematol.2023.282734. Haematologica. 2024. PMID: 37226713 Free PMC article. - AAV-mediated expression of HLA-G for the prevention of experimental ocular graft vs. host disease.
Nilles JP, Roberts D, Salmon JH, Song L, O'Dea C, Marjoram LT, Bower JJ, Hirsch ML, Gilger BC. Nilles JP, et al. Mol Ther Methods Clin Dev. 2023 Mar 24;29:227-235. doi: 10.1016/j.omtm.2023.03.012. eCollection 2023 Jun 8. Mol Ther Methods Clin Dev. 2023. PMID: 37090476 Free PMC article. - Ocular graft-versus-host disease (oGVHD): From A to Z.
Soleimani M, Mahdavi Sharif P, Cheraqpour K, Koganti R, Masoumi A, Baharnoori SM, Salabati M, Djalilian AR. Soleimani M, et al. Surv Ophthalmol. 2023 Jul-Aug;68(4):697-712. doi: 10.1016/j.survophthal.2023.02.006. Epub 2023 Mar 2. Surv Ophthalmol. 2023. PMID: 36870423 Free PMC article. Review.
References
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. - PubMed
- Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. - PubMed
- Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. - PubMed
- Data Collection Forms and Information for Measuring Disease Response in Chronic GvHD-related Clinical Trials: ASBMT Chronic GvHD Consensus Project. http://www.asbmt.org/displaycommon.cfm?an=1&subarticlenbr=29.
Publication types
MeSH terms
Grants and funding
- CA118953/CA/NCI NIH HHS/United States
- CA163438/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- U54 CA163438/CA/NCI NIH HHS/United States
- R01 CA118953/CA/NCI NIH HHS/United States
- U01 CA118953/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical