Global patterns of tissue-specific alternative polyadenylation in Drosophila - PubMed (original) (raw)
. 2012 Mar 29;1(3):277-89.
doi: 10.1016/j.celrep.2012.01.001.
Pedro Miura, Jakub O Westholm, Sol Shenker, Gemma May, Michael O Duff, Dayu Zhang, Brian D Eads, Joe Carlson, James B Brown, Robert C Eisman, Justen Andrews, Thomas Kaufman, Peter Cherbas, Susan E Celniker, Brenton R Graveley, Eric C Lai
Affiliations
- PMID: 22685694
- PMCID: PMC3368434
- DOI: 10.1016/j.celrep.2012.01.001
Global patterns of tissue-specific alternative polyadenylation in Drosophila
Peter Smibert et al. Cell Rep. 2012.
Erratum in
- Cell Rep. 2013 Mar 28;3(3):969
Abstract
We analyzed the usage and consequences of alternative cleavage and polyadenylation (APA) in Drosophila melanogaster by using >1 billion reads of stranded mRNA-seq across a variety of dissected tissues. Beyond demonstrating that a majority of fly transcripts are subject to APA, we observed broad trends for 3' untranslated region (UTR) shortening in the testis and lengthening in the central nervous system (CNS); the latter included hundreds of unannotated extensions ranging up to 18 kb. Extensive northern analyses validated the accumulation of full-length neural extended transcripts, and in situ hybridization indicated their spatial restriction to the CNS. Genes encoding RNA binding proteins (RBPs) and transcription factors were preferentially subject to 3' UTR extensions. Motif analysis indicated enrichment of miRNA and RBP sites in the neural extensions, and their termini were enriched in canonical cis elements that promote cleavage and polyadenylation. Altogether, we reveal broad tissue-specific patterns of APA in Drosophila and transcripts with unprecedented 3' UTR length in the nervous system.
Figures
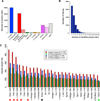
Figure 1. Poly(A)-Spanning RNA-Seq Reads Reveal Tissue-Specific Differences in 3′ UTR Length
(A) Distribution of poly(A) sites, minimum two reads per cluster, with respect to gene annotations. AS, antisense. (B) Distribution of 3′ UTR isoforms per gene. A cluster of two or more poly(A)-spanning reads downstream of a stop codon is classified as a poly(A)-supported 3′ end. (C) Distribution of 3′ UTR median length (using minimum two reads per cluster) with respect to FlyAtlas gene classifications. For “longest measured 3′ UTR” and “shortest measured 3′ UTRs,” poly(A)-spanning reads downstream of annotated 3′ ends and before the adjacent gene, and reads upstream of the annotated 3′ end, but after the stop codon were attributed to that gene. Lengths of longest and shortest 3′ UTRs from the FlyBase annotations of the same genes are shown for comparison. Note that nervous system tissues (highlighted with the red asterisks) have the longest 3′ UTRs as predicted by the poly(A)-spanning reads, whereas testis has the shortest 3′ UTRs (green asterisk); ovaries (highlighted with a black asterisk) have 3′ UTRs of intermediate length. The numbers after the tissue indicate the number of genes in each category. Acc, male accessory gland; prefix “L”, larval tissue; SG, salivary gland; Spt-M, mated spermatheca; Spt-V, virgin spermatheca; S2, S2 cells; TAG, thoracicoabdominal ganglion.
Figure 2. The Testis Transcriptome Is Biased toward Proximal Poly(A) Site Usage
(A–D) RNA-seq tracks for representative genes expressed in both male and female gonads, that exhibit evidence for truncated 3′ UTRs in testis compared to ovary. Additional examples can be found in Figure S1. The black regions correspond to mapped RNA-seq reads from each tissue, green indicates poly(A)-spanning reads pooled from 29 poly(A)-enriched RNA-seq libraries corresponding to the strand on which the gene of interest is expressed. The gray regions in (B) correspond to reads from the neighboring gene transcribed on the opposite strand. In (A–D), RT-PCR was performed using a common forward primer (black arrows) and UTR-specific reverse primers (blue arrows). To the right of the RNA-seq data are ethidium bromide stained gels, in which lower molecular weight bands derive from both long and short 3′ UTR isoforms, whereas higher molecular weight bands are specific to long 3′ UTR isoforms. Note the reduced levels of distal APA isoforms in testis. (E) Schematic for quantification of alternative length 3′ UTRs in ovary and testis by qRT-PCR. (F) qRT-PCR demonstrates 3′ UTR shortening in testis compared to ovaries for oaf, polo, slmb, e(r), and lack. Relative RNA levels are presented as ratio of total/long. Data are represented as mean ± SEM.
Figure 3. Unusually Long 3′ UTR Extensions in Neural Genes
(A) Distribution of 3′ UTR lengths of 383 transcripts that exhibit neural lengthening, relative to the longest annotated 3′ UTRs of all FlyBase 5.32 genes. (B) Focusing on the tail of the distribution, the neural 3′ UTR extensions comprise most of the longest 3′ UTRs in the Drosophila transcriptome. (C and D) RNA-seq tracks from indicated tissues (C) and transcript models for brat (D). A splice variant that alters the last few amino acids of Brat was apparent from RNA-seq data (gray), but this isoform was not differentially expressed. (E) In situ hybridization revealed that both proximal and distal probes detect CNS expression in germband retracted embryos and late stage embryos, but that the proximal probe alone detects maternal deposition and blastoderm expression in two stripes along the anterior-posterior axis. (F and G) RNA-seq tracks from indicated tissues (F) and gene models for mei-P26 (G). (H) Semiquantitative RT-PCR indicates that RNA corresponding to the 3′ end of the coding sequence and proximal 3′ UTR can be readily detected in all tissues and embryonic time-points examined, whereas an amplicon ~8 kb into the 3′ UTR was predominantly detected in heads and late stage embryos. (I) In situ hybridization reveals that both proximal and distal probes detect late stage expression in the brain and ventral nerve cord, but that the proximal probe alone detects maternal deposition at stage 2, posterior expression at stage 5, and ubiquitous expression at stage 14.
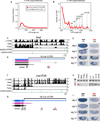
Figure 4. Northern Analysis Validates Stable Transcripts Corresponding to Strongly Distal APA Isoforms in Drosophila Heads
(A) Comparison of proximal “universal” probes and distal “extension” probes for a selection of exceptionally long inferred APA isoforms. In each case, a similarly sized band(s) is detected only in head samples with the paired probes (arrows), demonstrating that they connect a common 3′ UTR-extended transcript. In many cases, the sizes of these discrete lengthened transcripts far exceed the largest molecular weight marker on the RNA ladder (9 kb). (B) Comparison of proximal universal probes and distal extension probes for mei-P26. Note the 3′ UTR lengthening in head and 3′ UTR shortening in testis relative to body/ovary samples. (C) Examples of APA transcripts exhibiting neural extension (arrows) and testis shortening (arrowheads), often with intermediate-sized transcripts in body and/or ovary. (D) A broad selection of other transcripts that exhibit 3′ UTR-extended isoforms predominantly or exclusively in head (arrows), with the exception of imp. For bands that were outside the range of the RNA ladder, the estimated size of the longest 3′ UTR isoform in head was calculated based on RNA-seq coverage from head library and existing RefSeq mRNA annotations: shep, 9.4 kb; cut, 11.6 kb; brat, 11.7 kb; mei-P26, 22.9kb; bol, 10.9 kb; orb, 8.9 kb; sm, 12.7 kb; pum, 10.1 kb; msi, 10.9 kb; scrt, 9.1 kb; wech, 7.6 kb; imp, 11.8 kb; cpo, 9.3 kb; bru-3, 9.6 kb.
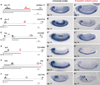
Figure 5. In Situ Hybridization Reveals Distinctive CNS Expression of Distal APA Isoforms
Schematics to the left include gene models with 3′ UTR extensions in head RNA-seq data relative to another tissue. H, head; O, ovary; 10–12 and 12–14, 10–12 and 12–14 hr embryos. The locations of proximal (black) and distal (red) in situ probes are indicated. The gene of interest is displayed in a plus strand orientation, and the name and orientation of the neighboring downstream gene is indicated. Panels with single prime labels show in situ hybridization patterns detected with a universal probe designed to detect all transcripts from the gene of interest. Panels with double prime labels show in situ hybridization patterns of probes specific for the extended isoforms. (A) khc-73 illustrates a gene with CNS staining detected with both universal and extended probes. (B) The universal bru-3 probe stains visceral muscle (VM) and CNS, whereas its extension probe stains only CNS. (C–E) fne (C), scrt (D), and elav (E) are pan-neuronally expressed in the peripheral nervous system(PNS) and CNS, yet their distal APA isoformsare specific to the CNS. (F) mub is an example of a neural transcript where the extension probe specifically detects expression in subregions of the embryonic brain (arrow) compared to the universal probe that detects expression throughout the embryonic brain and CNS.
Figure 6. Quality and Conservation of Alternative Poly(A) Sites and miRNA Sites
(A and B) Average 3′ UTR lengths of the proximal and distal transcript isoforms for genes exhibiting CNS 3′ UTR lengthening (A) and testis 3′ UTR shortening (B). (C and D) Sequence motifs involved in polyadenylation around poly(A) sites identified by poly(A)-spanning reads: Canonical PAS (in blue), GU-rich DSE (in green) and variant PAS (in gray). (C) For CNS extended genes, distal sites are enriched for the canonical PAS compared to proximal sites (Fisher’s exact test p value = 4.9E-16), whereas the frequency at the intermediate poly(A) sites lies in between. The distal poly(A) sites are also enriched for the DSE motif, compared to the proximal and intermediate poly(A) sites (Fisher’s exact test p value = 7.2E-4). All poly(A) sites show similar collective levels of variant PAS; see also Figure S5 for analysis of individual PAS variants. (D) Testis APA transcripts display similar patterns of motif occurrence and conservation as the CNS transcripts (enrichment of AAUAAA in distal versus proximal sites, Fisher’s exact test p value = 2.6E-4). (E) PhastCons conservation scores in the vicinity of poly(A) sites. Proximal and intermediate CNS poly(A) sites have intermediate conservation levels whereas distal sites have a region of high conservation just upstream of the poly(A) site and a region of low conservation downstream of the poly(A) site. (F) Proximal poly(A) sites in testis transcripts have intermediate conservation levels whereas the distal sites have a region of high conservation just upstream of the poly(A) site and region of low conservation downstream of the poly(A) site. (G) Cumulative distribution of numbers of miRNA target sites across all Drosophila 3′ UTRs, compared with the proximal and distal APA 3′ UTR variants of the 383 transcripts exhibiting neural lengthening. The distal extensions bear significantly more miRNA binding sites than Drosophila 3′ UTRs do in general. (H) Cumulative distribution of miRNA target site numbers in the 100 testis APA transcripts. (I) Motifs conserved above background in 3′ UTR extensions of neural transcripts. Shown are the top 6-mer motifs conserved above background in greater than or equal to seven Drosophilid species among the 383 3′ UTR extensions of neural transcripts. Many sites correspond to miRNA seeds or neural RBP sites (e.g., Pumilio and U-rich sequences that may potentially include Elav binding sites). A complete list of 6-mer and 7-mer motifs conserved above background are shown in Table S3. S/N, signal/noise ratio.
Similar articles
- Landscape and evolution of tissue-specific alternative polyadenylation across Drosophila species.
Sanfilippo P, Wen J, Lai EC. Sanfilippo P, et al. Genome Biol. 2017 Nov 30;18(1):229. doi: 10.1186/s13059-017-1358-0. Genome Biol. 2017. PMID: 29191225 Free PMC article. - Overlapping Activities of ELAV/Hu Family RNA Binding Proteins Specify the Extended Neuronal 3' UTR Landscape in Drosophila.
Wei L, Lee S, Majumdar S, Zhang B, Sanfilippo P, Joseph B, Miura P, Soller M, Lai EC. Wei L, et al. Mol Cell. 2020 Oct 1;80(1):140-155.e6. doi: 10.1016/j.molcel.2020.09.007. Mol Cell. 2020. PMID: 33007254 Free PMC article. - Molecular Regulation of Alternative Polyadenylation (APA) within the Drosophila Nervous System.
Vallejos Baier R, Picao-Osorio J, Alonso CR. Vallejos Baier R, et al. J Mol Biol. 2017 Oct 27;429(21):3290-3300. doi: 10.1016/j.jmb.2017.03.028. Epub 2017 Mar 31. J Mol Biol. 2017. PMID: 28366829 Free PMC article. - Implications of polyadenylation in health and disease.
Curinha A, Oliveira Braz S, Pereira-Castro I, Cruz A, Moreira A. Curinha A, et al. Nucleus. 2014;5(6):508-19. doi: 10.4161/nucl.36360. Epub 2014 Oct 31. Nucleus. 2014. PMID: 25484187 Free PMC article. Review. - Alternative polyadenylation in the nervous system: to what lengths will 3' UTR extensions take us?
Miura P, Sanfilippo P, Shenker S, Lai EC. Miura P, et al. Bioessays. 2014 Aug;36(8):766-77. doi: 10.1002/bies.201300174. Epub 2014 Jun 5. Bioessays. 2014. PMID: 24903459 Free PMC article. Review.
Cited by
- ELAV links paused Pol II to alternative polyadenylation in the Drosophila nervous system.
Oktaba K, Zhang W, Lotz TS, Jun DJ, Lemke SB, Ng SP, Esposito E, Levine M, Hilgers V. Oktaba K, et al. Mol Cell. 2015 Jan 22;57(2):341-8. doi: 10.1016/j.molcel.2014.11.024. Epub 2014 Dec 24. Mol Cell. 2015. PMID: 25544561 Free PMC article. - Alternative Polyadenylation in Triple-Negative Breast Tumors Allows NRAS and c-JUN to Bypass PUMILIO Posttranscriptional Regulation.
Miles WO, Lembo A, Volorio A, Brachtel E, Tian B, Sgroi D, Provero P, Dyson N. Miles WO, et al. Cancer Res. 2016 Dec 15;76(24):7231-7241. doi: 10.1158/0008-5472.CAN-16-0844. Epub 2016 Oct 10. Cancer Res. 2016. PMID: 27758885 Free PMC article. - DNA damage induces targeted, genome-wide variation of poly(A) sites in budding yeast.
Graber JH, Nazeer FI, Yeh PC, Kuehner JN, Borikar S, Hoskinson D, Moore CL. Graber JH, et al. Genome Res. 2013 Oct;23(10):1690-703. doi: 10.1101/gr.144964.112. Epub 2013 Jun 20. Genome Res. 2013. PMID: 23788651 Free PMC article. - ELAV mediates 3' UTR extension in the Drosophila nervous system.
Hilgers V, Lemke SB, Levine M. Hilgers V, et al. Genes Dev. 2012 Oct 15;26(20):2259-64. doi: 10.1101/gad.199653.112. Epub 2012 Sep 27. Genes Dev. 2012. PMID: 23019123 Free PMC article. - Alternative polyadenylation coupled to transcription initiation: Insights from ELAV-mediated 3' UTR extension.
Hilgers V. Hilgers V. RNA Biol. 2015;12(9):918-21. doi: 10.1080/15476286.2015.1060393. RNA Biol. 2015. PMID: 26158379 Free PMC article. Review.
References
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HG004271/HG/NHGRI NIH HHS/United States
- U01-HG004261/HG/NHGRI NIH HHS/United States
- RC2-HG005639/HG/NHGRI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01-GM083300/GM/NIGMS NIH HHS/United States
- U01-HB004271/HB/NHLBI NIH HHS/United States
- U01 HG004261/HG/NHGRI NIH HHS/United States
- R01 GM083300/GM/NIGMS NIH HHS/United States
- RC2 HG005639/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases