Nitric oxide formation versus scavenging: the red blood cell balancing act - PubMed (original) (raw)
Review
Nitric oxide formation versus scavenging: the red blood cell balancing act
Benjamin Y Owusu et al. J Physiol. 2012.
Abstract
Nitric oxide (NO) is a key modulator of vascular homeostasis controlling critical functions related to blood flow, respiration, cell death and proliferation, and protecting the vasculature from pro-inflammatory and coagulative stresses. Inhibition of NO formation, and/or diversion of NO away from its physiological signalling targets lead to dysregulated NO bioavailability, a hallmark of numerous vascular and pulmonary diseases. Current concepts suggest that the balance between NO formation and NO scavenging is critical in disease development, with the corollary being that redressing the balance offers a target for therapeutic intervention. Evidence presented over the last two decades has seen red blood cells (RBCs) and haemoglobin specifically emerge as prominent effectors in this paradigm. In this symposium review article, we discuss recent insights into the mechanisms by which RBCs may modulate the balance between NO-formation and inhibition. We discuss how these mechanisms may become dysfunctional to cause disease, highlight key questions that remain, and discuss the potential impact of these insights on therapeutic opportunities.
Figures
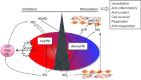
Figure 1. Current models for how RBCs can inhibit and stimulate NO signalling
Shown is how haemoglobin oxygen sensing may be coupled to NO scavenging (by intact or cell-free haemoglobin), or formation (from deoxyhaemoglobin-mediated nitrite reduction or ATP release and subsequent eNOS activation) to control NO bioavailability in the vasculature. Also shown is the concept that nitrite reduction to elicit NO signalling during hypoxia may occur by tissues independent of RBCs, but RBCs can affect this process by controlling nitrite concentration via oxidation to nitrate.
Similar articles
- Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease.
Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Crawford JH, et al. Blood. 2004 Sep 1;104(5):1375-82. doi: 10.1182/blood-2004-03-0880. Epub 2004 May 18. Blood. 2004. PMID: 15150083 - Nitric oxide scavenging by hemoglobin regulates hypoxic pulmonary vasoconstriction.
Deem S. Deem S. Free Radic Biol Med. 2004 Mar 15;36(6):698-706. doi: 10.1016/j.freeradbiomed.2003.11.025. Free Radic Biol Med. 2004. PMID: 14990350 Review. - Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction.
Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Nagababu E, et al. J Biol Chem. 2003 Nov 21;278(47):46349-56. doi: 10.1074/jbc.M307572200. Epub 2003 Sep 2. J Biol Chem. 2003. PMID: 12952953 - Regulation of oxygen delivery by the reaction of nitrite with RBCs under hypoxic conditions.
Rifkind JM, Salgado MT, Cao Z. Rifkind JM, et al. Adv Exp Med Biol. 2012;737:183-9. doi: 10.1007/978-1-4614-1566-4_27. Adv Exp Med Biol. 2012. PMID: 22259100 Free PMC article. No abstract available. - Red blood cells and hemoglobin in hypoxic pulmonary vasoconstriction.
Deem S. Deem S. Adv Exp Med Biol. 2006;588:217-31. doi: 10.1007/978-0-387-34817-9_19. Adv Exp Med Biol. 2006. PMID: 17089892 Review.
Cited by
- Pulsed ultrasound enhances the delivery of nitric oxide from bubble liposomes to ex vivo porcine carotid tissue.
Sutton JT, Raymond JL, Verleye MC, Pyne-Geithman GJ, Holland CK. Sutton JT, et al. Int J Nanomedicine. 2014 Oct 6;9:4671-83. doi: 10.2147/IJN.S63850. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25336947 Free PMC article. - Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response.
Horobin JT, Sabapathy S, Kuck L, Simmonds MJ. Horobin JT, et al. Life (Basel). 2021 Jan 8;11(1):36. doi: 10.3390/life11010036. Life (Basel). 2021. PMID: 33429979 Free PMC article. - Contribution of non-endothelium-dependent substances to exercise hyperaemia: are they O(2) dependent?
Marshall JM, Ray CJ. Marshall JM, et al. J Physiol. 2012 Dec 15;590(24):6307-20. doi: 10.1113/jphysiol.2012.240721. Epub 2012 Oct 8. J Physiol. 2012. PMID: 23045341 Free PMC article. Review. - Stimulation of Erythrocyte Soluble Guanylyl Cyclase Induces cGMP Export and Cardioprotection in Type 2 Diabetes.
Jiao T, Collado A, Mahdi A, Tengbom J, Tratsiakovich Y, Milne GT, Alvarsson M, Lundberg JO, Zhou Z, Yang J, Pernow J. Jiao T, et al. JACC Basic Transl Sci. 2023 Aug 21;8(8):907-918. doi: 10.1016/j.jacbts.2023.02.017. eCollection 2023 Aug. JACC Basic Transl Sci. 2023. PMID: 37719424 Free PMC article. - An Overview of NO Signaling Pathways in Aging.
Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. Pourbagher-Shahri AM, et al. Molecules. 2021 Jul 27;26(15):4533. doi: 10.3390/molecules26154533. Molecules. 2021. PMID: 34361685 Free PMC article. Review.
References
- Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H2068–2074. - PubMed
- Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–39032. - PubMed
- Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources