Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat - PubMed (original) (raw)
Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat
Ana P Abdala et al. J Physiol. 2012.
Abstract
The peripheral chemoreflex is known to be enhanced in individuals with hypertension. In pre-hypertensive (PH) and adult spontaneously hypertensive rats (SHRs) carotid body type I (glomus) cells exhibit hypersensitivity to chemosensory stimuli and elevated sympathoexcitatory responses to peripheral chemoreceptor stimulation. Herein, we eliminated carotid body inputs in both PH-SHRs and SHRs to test the hypothesis that heightened peripheral chemoreceptor activity contributes to both the development and maintenance of hypertension. The carotid sinus nerves were surgically denervated under general anaesthesia in 4- and 12-week-old SHRs. Control groups comprised sham-operated SHRs and aged-matched sham-operated and carotid sinus nerve denervated Wistar rats. Arterial blood pressure was recorded chronically in conscious, freely moving animals. Successful carotid sinus nerve denervation (CSD) was confirmed by testing respiratory responses to hypoxia (10% O(2)) or cardiovascular responses to i.v. injection of sodium cyanide. In the SHR, CSD reduced both the development of hypertension and its maintenance (P<0.05) and was associated with a reduction in sympathetic vasomotor tone (as revealed by frequency domain analysis and reduced arterial pressure responses to administration of hexamethonium; P<0.05 vs. sham-operated SHR) and an improvement in baroreflex sensitivity. No effect on blood pressure was observed in sham-operated SHRs or Wistar rats. In conclusion, carotid sinus nerve inputs from the carotid body are, in part, responsible for elevated sympathetic tone and critical for the genesis of hypertension in the developing SHR and its maintenance in later life.
Figures
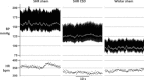
Figure 1. CSD is anti-hypertensive in the developing spontaneously hypertensive rat (SHR)
Representative recordings of the arterial blood pressure (BP) and heart rate (HR) in 13-week-old CSD SHR, sham-operated SHR and sham-operated Wistar rat. Carotid sinus nerves were denervated at 4 weeks of age before SHRs become hypertensive.
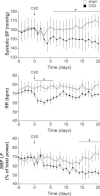
Figure 2. Anti-hypertensive effect of CSD in the pre-hypertensive (PH)-SHRs is associated with lowered sympathetic vasomotor tone
A, CSD in PH-SHRs delays the development and reduces the magnitude of hypertension. Following CSD at 4 weeks of age, SHRs remained normotensive at 11 weeks old while sham-operated SHRs developed hypertension. By 13 weeks of age, CSD SHRs were hypertensive (P < 0.05 vs. Wistar rats) but systolic, diastolic, mean and pulse pressures were all lower compared with those in sham-operated SHRs. There was no effect of CSD on heart rate (HR) but respiratory rate (RR) was reduced in the PH-SHR to a level comparable to normotensive rats. B, in PH-SHRs, sympathetic vasomotor tone was reduced as revealed by a reduction in very low frequency (VLF) and LF:HF ratio of systolic pressure (P < 0.05 vs. sham-operated SHR). The low frequency (LF) component in SHR was not affected by the CSD. In contrast, CSD Wistar rats showed higher power of the VLF component (vs. sham-operated Wistar rats) and lower power in the HF component (vs. sham-operated Wistar rats), whilst the LF component and LF:HF ratio were unaffected. Data represent mean ± SEM. Effect of CSD within each strain (n = 6 per group; *P < 0.05, **P < 0.01, ***P < 0.01) and between strains (+P < 0.05, ++P < 0.01, +++P < 0.001) were analysed with two-way ANOVA.
Figure 3. In established hypertension, CSD in SHRs reduces arterial pressure, respiratory rate and the power of low frequency systolic pressure variability
Time profile of changes in systolic pressure (BP), respiratory rate (RR, breaths min−1) and low-frequency SBP variability (an indirect index of vasomotor tone) 5 days before and 21 days after CSD (arrows; n = 8) or sham surgery (n = 7) in the adult (12 week old) SHR. #P < 0.05.
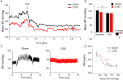
Figure 4. CSD in the adult SHR reduces sympathetic vasomotor tone and improves baroreceptor reflex sensitivity
A and B, blockade of autonomic ganglionic transmission produced a larger fall in arterial pressure in sham-operated SHR compared with that in the CSD SHR suggesting less vasomotor sympathetic tone in the latter group. C, arterial blood pressure (BP) responses during sodium cyanide injection (
i.v.
) to activate the peripheral chemoreceptors. Note that after CSD, there was no peripheral chemoreflex-mediated pressor and bradycardic responses (compared with sham-operated) indicative of an efficacious denervation. D, baroreflex function curve of heart rate (HR) vs. mean blood pressure (MBP) obtained using
i.v.
phenylephrine and sodium nitroprusside injections. Note the shift in the operating point to re-engage reflex function after CSD (red) compared with sham-operated (black). #P < 0.05.
Comment in
- The carotid body in cardiovascular disease: more chicken and egg than horse and cart?
Kumar P. Kumar P. J Physiol. 2012 Sep 1;590(17):4123. doi: 10.1113/jphysiol.2012.239921. J Physiol. 2012. PMID: 22962032 Free PMC article. No abstract available.
Similar articles
- Blood pressure, heart rate and arterial blood gas reactions to acute hypoxia in carotid body denervated spontaneously hypertensive rats.
Huckstorf C, Behm R, Habeck JO, Rückborn K, Franz U. Huckstorf C, et al. Biomed Biochim Acta. 1987;46(12):925-31. Biomed Biochim Acta. 1987. PMID: 3453075 - Variable role of carotid bodies in cardiovascular responses to exercise, hypoxia and hypercapnia in spontaneously hypertensive rats.
Pijacka W, Katayama PL, Salgado HC, Lincevicius GS, Campos RR, McBryde FD, Paton JFR. Pijacka W, et al. J Physiol. 2018 Aug;596(15):3201-3216. doi: 10.1113/JP275487. Epub 2018 Feb 12. J Physiol. 2018. PMID: 29313987 Free PMC article. - Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats.
Pijacka W, McBryde FD, Marvar PJ, Lincevicius GS, Abdala AP, Woodward L, Li D, Paterson DJ, Paton JF. Pijacka W, et al. J Physiol. 2016 Nov 1;594(21):6255-6266. doi: 10.1113/JP272708. Epub 2016 Sep 23. J Physiol. 2016. PMID: 27510951 Free PMC article. - Chemoreflexes--physiology and clinical implications.
Kara T, Narkiewicz K, Somers VK. Kara T, et al. Acta Physiol Scand. 2003 Mar;177(3):377-84. doi: 10.1046/j.1365-201X.2003.01083.x. Acta Physiol Scand. 2003. PMID: 12609009 Review. - Revelations about carotid body function through its pathological role in resistant hypertension.
Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz K. Paton JF, et al. Curr Hypertens Rep. 2013 Aug;15(4):273-80. doi: 10.1007/s11906-013-0366-z. Curr Hypertens Rep. 2013. PMID: 23828147 Free PMC article. Review.
Cited by
- Expression of group II and III mGluRs in the carotid body and its role in the carotid chemoreceptor response to acute hypoxia.
Zhao C, Li C, Zhao B, Liu Y. Zhao C, et al. Front Physiol. 2022 Sep 22;13:1008073. doi: 10.3389/fphys.2022.1008073. eCollection 2022. Front Physiol. 2022. PMID: 36213225 Free PMC article. - TRPV1 (Transient Receptor Potential Vanilloid 1) Cardiac Spinal Afferents Contribute to Hypertension in Spontaneous Hypertensive Rat.
Shanks J, de Morais SDB, Gao L, Zucker IH, Wang HJ. Shanks J, et al. Hypertension. 2019 Oct;74(4):910-920. doi: 10.1161/HYPERTENSIONAHA.119.13285. Epub 2019 Aug 19. Hypertension. 2019. PMID: 31422690 Free PMC article. - RNA Sequencing Reveals Novel Transcripts from Sympathetic Stellate Ganglia During Cardiac Sympathetic Hyperactivity.
Bardsley EN, Davis H, Ajijola OA, Buckler KJ, Ardell JL, Shivkumar K, Paterson DJ. Bardsley EN, et al. Sci Rep. 2018 Jun 5;8(1):8633. doi: 10.1038/s41598-018-26651-7. Sci Rep. 2018. PMID: 29872217 Free PMC article. - Sympathoactivation and rho-kinase-dependent baroreflex function in experimental renovascular hypertension with reduced kidney mass.
Pliquett RU, Benkhoff S, Jung O, Brandes RP. Pliquett RU, et al. BMC Physiol. 2014 Jun 19;14:4. doi: 10.1186/1472-6793-14-4. BMC Physiol. 2014. PMID: 24946879 Free PMC article. - Electrophysiological properties of laryngeal motoneurones in rats submitted to chronic intermittent hypoxia.
Moraes DJ, Machado BH. Moraes DJ, et al. J Physiol. 2015 Feb 1;593(3):619-34. doi: 10.1113/jphysiol.2014.283085. Epub 2015 Jan 5. J Physiol. 2015. PMID: 25433075 Free PMC article.
References
- Abdala APL, Marina N, Gourine AV, Paton JFR. Peripheral chemoreceptor inputs contribute to the development of high blood pressure in spontaneously hypertensive rats. Proc Physiol Soc. 2011a;23:PC22.
- Abdala APL, Paton JFR, Gourine AV. Peripheral chemoreceptor (PC) inputs contribute to the development of high blood pressure in spontaneously hypertensive rats (SHR) FASEB J. 2011b;25:640.10.
- Abdala AP, Schoorlemmer GH, Colombari E. Ablation of NK1 receptor bearing neurons in the nucleus of the solitary tract blunts cardiovascular reflexes in awake rats. Brain Res. 2006;1119:165–173. - PubMed
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. - PubMed
Publication types
MeSH terms
Grants and funding
- 095064/WT_/Wellcome Trust/United Kingdom
- FS/12/1/29080/BHF_/British Heart Foundation/United Kingdom
- R01 NS069220/NS/NINDS NIH HHS/United States
- RG/07/006/23634/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical