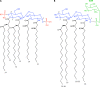Subversion of host recognition and defense systems by Francisella spp - PubMed (original) (raw)
Review
Subversion of host recognition and defense systems by Francisella spp
Crystal L Jones et al. Microbiol Mol Biol Rev. 2012 Jun.
Abstract
Francisella tularensis is a gram-negative intracellular pathogen and the causative agent of the disease tularemia. Inhalation of as few as 10 bacteria is sufficient to cause severe disease, making F. tularensis one of the most highly virulent bacterial pathogens. The initial stage of infection is characterized by the "silent" replication of bacteria in the absence of a significant inflammatory response. Francisella achieves this difficult task using several strategies: (i) strong integrity of the bacterial surface to resist host killing mechanisms and the release of inflammatory bacterial components (pathogen-associated molecular patterns [PAMPs]), (ii) modification of PAMPs to prevent activation of inflammatory pathways, and (iii) active modulation of the host response by escaping the phagosome and directly suppressing inflammatory pathways. We review the specific mechanisms by which Francisella achieves these goals to subvert host defenses and promote pathogenesis, highlighting as-yet-unanswered questions and important areas for future study.
Figures
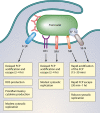
Fig 1
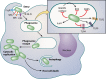
Stages of Francisella pathogenesis in the macrophage. Francisella can be detected by multiple macrophage receptors (see “Mechanisms of Entry and Fate of Intracellular Francisella” below) and is engulfed by a unique pseudopod loop mechanism. It then traffics to an early phagosome called the Francisella-containing phagosome (FCP). Francisella uses multiple mechanisms to evade host defenses in this harsh environment (inset). Francisella blocks the NADPH oxidase and also detoxifies reactive oxygen species (ROS). It can also resist the action of antimicrobial peptides (AMPs). Francisella does not signal through TLR4 but does activate TLR2 and may induce TLR9 signaling. Francisella then escapes the FCP to replicate within the cytosol. Subsequently, Francisella associates with autophagosomes, although the outcome of this interaction is unknown. Francisella can also induce host cell death.
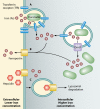
Fig 2
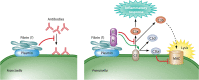
Complement Evasion by Francisella. Antibodies (Abs) can bind to the bacterial surface (left panel), leading to binding of complement component C3 (right panel), triggering activation of the complement cascade and generation of C3a, an inflammatory anaphylatoxin, and C3b. C3b ultimately leads to the formation of the membrane attack complex (MAC) and lysis of the bacterial cell. Francisella counteracts these defenses by binding host plasmin, which inhibits antibody binding (left panel) and thus complement activation. Francisella also binds host factor H, which inhibits C3 cleavage to C3a and C3b and instead acts with host factor I to create the complement-inhibitory molecules C3d and C3bi. This skews the complement cascade from promoting MAC formation and lysis to facilitating phagocytosis. Factor H binding may be promoted by fibrinogen, which is converted to fibrin and is known to bind plasmin, but this has yet to be demonstrated.
Fig 3
Shielding of inflammatory PAMPs in Francisella. Francisella modifies and limits the release of its inflammatory components (PAMPs) that can be recognized by pattern recognition receptors, such as TLRs, expressed by antigen-presenting cells (APC). Francisella bacterial lipoproteins (BLPs) and DNA (shown in red) are PAMPs with inflammatory activity, while the capsule, LPS, free lipid A, and membrane phospholipids do not elicit an inflammatory response. Francisella modifies its LPS and free lipid A such that these PAMPs do not activate host TLR4. It is unclear whether Francisella peptidoglycan (PGN) has inflammatory activity (represented in brown). Membrane proteins that are not BLPs and are not as a class considered PAMPs are shown in purple. The capsule and LPS O antigen provide resistance to antimicrobials such as complement and antimicrobial peptides, preventing damage to the bacterial membranes, which would result in the release of immunostimulatory BLPs and DNA. In addition to providing resistance to the damaging effects of antimicrobials, the capsule and O antigen may also serve as physical barriers to the release of PAMPs into the environment.
Fig 4
E. coli and Francisella lipid A structures. The structures of the E. coli lipid A moiety (A) and free lipid A from Francisella species (B) are shown. For both structures, the sugar backbone is highlighted in blue and acyl chains are represented in black, with numbers denoting length. Phosphate groups at the E. coli lipid A 4′ and 1 positions (absent from lipid A of full-length Francisella LPS) are highlighted in red. Francisella free lipid A contains the phosphate at the 1 position and is modified with galactosamine (GalN) (in green).
Fig 5
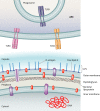
Intracellular fates of Francisella after uptake by different macrophage receptors. Antibody-opsonized Francisella is taken up via the FcγR, leading to increased ROS production and induction of proinflammatory cytokines, delayed FCP acidification and bacterial escape (2 to 4 h) from the phagosome, and only moderate levels of cytosolic replication. Uptake of serum (complement)-opsonized Francisella is mediated mainly by complement receptor 3 (CR3) and scavenger A receptors (SRA), which lead to slowed FCP acidification and phagosomal escape (2 to 4 h) and result in modest cytosolic replication. Lastly, uptake of unopsonized Francisella is mediated by the mannose receptor (MR) and surface-exposed nucleolin (SE-N), leading to rapid acidification (15 to 30 min) and escape from the FCP (30 min to 1 h) and robust cytosolic replication.
Fig 6
Competition for iron during Francisella infection. (A) Upon infection of macrophages, Francisella induces the upregulation and relocalization of the transferrin receptor (TfR) to the forming phagosome, facilitating an influx of iron into the host cell. (B) The host counteracts this by upregulating Dmt1 and ferroportin, which export iron from the phagosome to the cytosol and from the cytosol to the extracellular space, respectively. (C) Francisella, however, counters this by inducing host hepcidin, which binds ferroportin and causes it to be degraded. In total, this leads to an increase of intracellular iron available in the cytosol for Francisella replication.
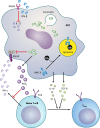
Fig 7
Subversion of adaptive immune responses by Francisella. Francisella infects antigen-presenting cells (APCs) and dampens their ability to produce proinflammatory cytokines. IL-12 is blocked via the induction of beta interferon (IFN-β). IFN-γ is produced by dendritic cells and T cells during Francisella infection, and it induces macrophages to kill the bacteria. Francisella blocks signal transduction through the IFN-γ receptor (IFNGR) by inhibiting Stat1 phosphorylation and through the induction of prostaglandin E2 (PGE2), which inhibits IFN-γ production by T cells. Francisella also hampers T cell activation by inducing the degradation of major histocompatibility complex class II (MHC II) (indicated by its absence from the cell surface, tagging with ubiquitin [Ub], and trafficking toward the lysosome) and directing APCs to produce the anti-inflammatory cytokines TGF-β and IL-10, which promote the development of regulatory T cells (Treg) that are able to suppress inflammation and cell-mediated immune responses.
Similar articles
- Mechanisms of Francisella tularensis intracellular pathogenesis.
Celli J, Zahrt TC. Celli J, et al. Cold Spring Harb Perspect Med. 2013 Apr 1;3(4):a010314. doi: 10.1101/cshperspect.a010314. Cold Spring Harb Perspect Med. 2013. PMID: 23545572 Free PMC article. Review. - Francisella tularensis subsp. tularensis induces a unique pulmonary inflammatory response: role of bacterial gene expression in temporal regulation of host defense responses.
Walters KA, Olsufka R, Kuestner RE, Cho JH, Li H, Zornetzer GA, Wang K, Skerrett SJ, Ozinsky A. Walters KA, et al. PLoS One. 2013 May 14;8(5):e62412. doi: 10.1371/journal.pone.0062412. Print 2013. PLoS One. 2013. PMID: 23690939 Free PMC article. - Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages.
Clemens DL, Lee BY, Horwitz MA. Clemens DL, et al. Infect Immun. 2004 Jun;72(6):3204-17. doi: 10.1128/IAI.72.6.3204-3217.2004. Infect Immun. 2004. PMID: 15155622 Free PMC article. - OpiA, a Type Six Secretion System Substrate, Localizes to the Cell Pole and Plays a Role in Bacterial Growth and Viability in Francisella tularensis LVS.
Cantlay S, Haggerty K, Horzempa J. Cantlay S, et al. J Bacteriol. 2020 Jun 25;202(14):e00048-20. doi: 10.1128/JB.00048-20. Print 2020 Jun 25. J Bacteriol. 2020. PMID: 32366588 Free PMC article. - Francisella tularensis travels a novel, twisted road within macrophages.
Santic M, Molmeret M, Klose KE, Abu Kwaik Y. Santic M, et al. Trends Microbiol. 2006 Jan;14(1):37-44. doi: 10.1016/j.tim.2005.11.008. Epub 2005 Dec 13. Trends Microbiol. 2006. PMID: 16356719 Review.
Cited by
- Type I interferon licenses enhanced innate recognition and transcriptional responses to Franciscella tularensis live vaccine strain.
Richard K, Vogel SN, Perkins DJ. Richard K, et al. Innate Immun. 2016 Jul;22(5):363-72. doi: 10.1177/1753425916650027. Epub 2016 May 26. Innate Immun. 2016. PMID: 27231145 Free PMC article. - Francisella tularensis LVS induction of prostaglandin biosynthesis by infected macrophages requires specific host phospholipases and lipid phosphatases.
Navratil AR, Brummett AM, Bryan JD, Woolard MD. Navratil AR, et al. Infect Immun. 2014 Aug;82(8):3299-311. doi: 10.1128/IAI.02060-14. Epub 2014 May 27. Infect Immun. 2014. PMID: 24866789 Free PMC article. - Isolation of _F. novicida_-Containing Phagosome from Infected Human Monocyte Derived Macrophages.
Marecic V, Shevchuk O, Ozanic M, Mihelcic M, Steinert M, Jurak Begonja A, Abu Kwaik Y, Santic M. Marecic V, et al. Front Cell Infect Microbiol. 2017 Jul 5;7:303. doi: 10.3389/fcimb.2017.00303. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28725638 Free PMC article. - Comparative review of Francisella tularensis and Francisella novicida.
Kingry LC, Petersen JM. Kingry LC, et al. Front Cell Infect Microbiol. 2014 Mar 13;4:35. doi: 10.3389/fcimb.2014.00035. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24660164 Free PMC article. Review. - Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium.
Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, Whitmire JK, Miao EA. Maltez VI, et al. Immunity. 2015 Nov 17;43(5):987-97. doi: 10.1016/j.immuni.2015.10.010. Epub 2015 Nov 10. Immunity. 2015. PMID: 26572063 Free PMC article.
References
- Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. 2005. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 174:3658–3667 - PubMed
- Amer LS, Bishop BM, van Hoek ML. 2010. Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem. Biophys. Res. Commun. 396:246–251 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI087673/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- U54-AI057157/AI/NIAID NIH HHS/United States
- R56-AI087673/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous