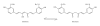Pleiotropic protective effects of phytochemicals in Alzheimer's disease - PubMed (original) (raw)
Review
Pleiotropic protective effects of phytochemicals in Alzheimer's disease
Sergio Davinelli et al. Oxid Med Cell Longev. 2012.
Abstract
Alzheimer's disease (AD) is a severe chronic neurodegenerative disorder of the brain characterised by progressive impairment in memory and cognition. In the past years an intense research has aimed at dissecting the molecular events of AD. However, there is not an exhaustive knowledge about AD pathogenesis and a limited number of therapeutic options are available to treat this neurodegenerative disease. Consequently, considering the heterogeneity of AD, therapeutic agents acting on multiple levels of the pathology are needed. Recent findings suggest that phytochemicals compounds with neuroprotective features may be an important resources in the discovery of drug candidates against AD. In this paper we will describe some polyphenols and we will discuss their potential role as neuroprotective agents. Specifically, curcumin, catechins, and resveratrol beyond their antioxidant activity are also involved in antiamyloidogenic and anti-inflammatory mechanisms. We will focus on specific molecular targets of these selected phytochemical compounds highlighting the correlations between their neuroprotective functions and their potential therapeutic value in AD.
Figures
Figure 1
Chemical structures of Curcumin. Curcumin belongs to the class of curcuminoids and the presence of double bonds increases its potency and reactivity. The phytochemical curcumin undergoes keto-enol tautomerism.
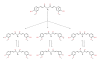
Figure 2
Reaction mechanism of curcumin with free radicals. The reactions produce phenoxyl radicals and carbon-centered radical at the methylene CH2 group.
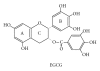
Figure 3
Chemical structure of (−)-Epigallocatechin-3-gallate. EGCG contains three heterocyclic rings, A, B, and C, and the free radical scavenging property of EGCG is attributed to the presence of trihydroxyl group on the B ring and the gallate moiety at the 3′ position in the C ring.
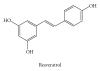
Figure 4
Chemical structure of resveratrol. The 4′-OH in resveratrol provides its chemical and biological features.The transfer of protons or hydrogen atoms to reactive species appears to be crucial to its antioxidant mechanism.
Similar articles
- Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders.
Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Scapagnini G, et al. Mol Neurobiol. 2011 Oct;44(2):192-201. doi: 10.1007/s12035-011-8181-5. Epub 2011 Apr 19. Mol Neurobiol. 2011. PMID: 21499987 Free PMC article. Review. - Natural antioxidants in Alzheimer's disease.
Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V. Mancuso C, et al. Expert Opin Investig Drugs. 2007 Dec;16(12):1921-31. doi: 10.1517/13543784.16.12.1921. Expert Opin Investig Drugs. 2007. PMID: 18042001 Review. - Naturally occurring phytochemicals for the prevention of Alzheimer's disease.
Kim J, Lee HJ, Lee KW. Kim J, et al. J Neurochem. 2010 Mar;112(6):1415-30. doi: 10.1111/j.1471-4159.2009.06562.x. Epub 2009 Dec 26. J Neurochem. 2010. PMID: 20050972 Review. - Polyphenols: key issues involved in chemoprevention of prostate cancer.
Cimino S, Sortino G, Favilla V, Castelli T, Madonia M, Sansalone S, Russo GI, Morgia G. Cimino S, et al. Oxid Med Cell Longev. 2012;2012:632959. doi: 10.1155/2012/632959. Epub 2012 May 28. Oxid Med Cell Longev. 2012. PMID: 22690272 Free PMC article. Review. - Neuroprotective potentials of Lead phytochemicals against Alzheimer's disease with focus on oxidative stress-mediated signaling pathways: Pharmacokinetic challenges, target specificity, clinical trials and future perspectives.
Ayaz M, Mosa OF, Nawaz A, Hamdoon AAE, Elkhalifa MEM, Sadiq A, Ullah F, Ahmed A, Kabra A, Khan H, Murthy HCA. Ayaz M, et al. Phytomedicine. 2024 Feb;124:155272. doi: 10.1016/j.phymed.2023.155272. Epub 2023 Dec 12. Phytomedicine. 2024. PMID: 38181530 Review.
Cited by
- Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway.
Tiwari SK, Agarwal S, Tripathi A, Chaturvedi RK. Tiwari SK, et al. Mol Neurobiol. 2016 Jul;53(5):3010-3029. doi: 10.1007/s12035-015-9197-z. Epub 2015 May 12. Mol Neurobiol. 2016. PMID: 25963729 - Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges.
Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Davinelli S, et al. Immun Ageing. 2016 Apr 14;13:16. doi: 10.1186/s12979-016-0070-3. eCollection 2016. Immun Ageing. 2016. PMID: 27081392 Free PMC article. Review. - Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview.
Libro R, Giacoppo S, Soundara Rajan T, Bramanti P, Mazzon E. Libro R, et al. Molecules. 2016 Apr 21;21(4):518. doi: 10.3390/molecules21040518. Molecules. 2016. PMID: 27110749 Free PMC article. Review. - Zataria multiflora could improve hippocampal tau protein and TNFα levels and cognitive behavior defects in a rat model of Alzheimer's disease.
Ahmadi M, Taherianfard M, Shomali T. Ahmadi M, et al. Avicenna J Phytomed. 2019 Sep-Oct;9(5):465-473. Avicenna J Phytomed. 2019. PMID: 31516860 Free PMC article.
References
- Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid β-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. Journal of Alzheimer’s Disease. 2002;4(3):193–201. - PubMed
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. Journal of Neuroimmunology. 2007;184(1-2):69–91. - PubMed
- Wollen KA. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Alternative Medicine Review. 2010;15(3):223–244. - PubMed
- Williams R. Biomarkers: warning signs. Nature. 2011;475(7355):S5–S7. - PubMed
- Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. Journal of Agricultural and Food Chemistry. 2008;56(13):4855–4873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous