miR-132 enhances dendritic morphogenesis, spine density, synaptic integration, and survival of newborn olfactory bulb neurons - PubMed (original) (raw)
miR-132 enhances dendritic morphogenesis, spine density, synaptic integration, and survival of newborn olfactory bulb neurons
Manavendra Pathania et al. PLoS One. 2012.
Abstract
An array of signals regulating the early stages of postnatal subventricular zone (SVZ) neurogenesis has been identified, but much less is known regarding the molecules controlling late stages. Here, we investigated the function of the activity-dependent and morphogenic microRNA miR-132 on the synaptic integration and survival of olfactory bulb (OB) neurons born in the neonatal SVZ. In situ hybridization revealed that miR-132 expression occurs at the onset of synaptic integration in the OB. Using in vivo electroporation we found that sequestration of miR-132 using a sponge-based strategy led to a reduced dendritic complexity and spine density while overexpression had the opposite effects. These effects were mirrored with respective changes in the frequency of GABAergic and glutamatergic synaptic inputs reflecting altered synaptic integration. In addition, timely directed overexpression of miR-132 at the onset of synaptic integration using an inducible approach led to a significant increase in the survival of newborn neurons. These data suggest that miR-132 forms the basis of a structural plasticity program seen in SVZ-OB postnatal neurogenesis. miR-132 overexpression in transplanted neurons may thus hold promise for enhancing neuronal survival and improving the outcome of transplant therapies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
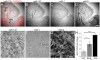
Figure 1. miR-132 is expressed in newborn SVZ neurons at the onset of synaptic integration.
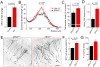
(A–D) In situ hybridization images of miR-132 with TOPRO-3 (red) overlay (red, A), miR-132 (B), miR-1 (C), and miR-9 (D) in a sagittal section containing the SVZ, RMS and OB. (E–G) Higher magnification of miR-132, miR-1 and miR-9 images in the granule cell layer (GCL). Scale bars: 100 µm (A–D) and 30 µm (E–F). The image in (E) comes from the boxed region in (A). (H) Bar graphs of miR-132 qRT-PCR fold changes in the RMSOB and GCL compared to the SVZ.
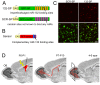
Figure 2. Validation of the specificity of miR-132 sponge vectors and experimental diagram.
(A) Schematic of vectors encoding GFP containing 20 tandem, miR-132-binding sites in its 3′UTR (orange bars) to “sponge” out miR-132 (132-SP) and a control vector encoding mutant GFP containing 20 random sites in its 3′UTR (SCR9-SP). (B) Schematic of a sensor vector encoding RFP containing perfectly complementary miR-132 binding sites (blue bars). (C) Confocal images of Neuro-2A cells transfected with the sensor vector and SCR-SP or 132-SP. Scale bar: 70 µm. (D) Diagram of our experimental paradigm. DNA constructs were introduced into the lateral ventricle of P0–P1 pups for electroporation into neural progenitor cells. 4–6 weeks post-electroporation, fluorescently tagged, synaptically integrated newborn neurons were analyzed.
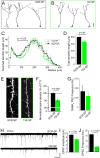
Figure 3. miR-132 sequestration in vivo truncates dendritic development leading to synaptic input deprivation.
(A and B) Representative reconstructions of SCR-SP- (A) and 132-SP-expressing (B, green) newborn neurons at 6 wpe in the GCL. (C and D) Plots of the summed dendritic length (C) and bar graphs of the total dendritic length (D) of SCR-SP-(black) and 132-SP-expressing (B, green) newborn neurons (n = 22 and 25 neurons, respectively). (E) Confocal images of spines in fluorescent neurons containing: SCR-132 (black) or 132-SP (green). (F) Comparison of normalized spine density. N = 3 mice for each condition. (G) Bar graph of the mean frequency of GABAA PSCs in SCR-SP (black) and 132-SP (green) neurons (n = 18 and 16 neurons, respectively). (H) Representative examples of EPSCs in neurons containing SCR-SP and 132-SP. Scale bar: 10 pA/30 s. (I and J) Bar graphs of the mean amplitude (I) and frequency (J) in neurons containing SCR-SP (black, n = 11 cells) and 132-SP (green, n = 11 cells). Scale bar in A–B: 50 µm; in E: 10 µm.
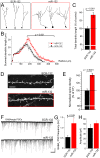
Figure 4. miR-132 overexpression promotes dendritic morphogenesis and synaptic integration in vivo.
(A and B) Representative reconstructions of SCR-132 (A) and miR-132-expressing (B, red) newborn neurons at 8 wpe in the GCL. (B) Plots of the summed dendritic length of SCR-132-(black) and miR-132 expressing (B, red) newborn neurons (n = 38 and 55 neurons, respectively). (C) Bar graphs of the percentage (%) of control for the total dendritic length of miR-132 overexpressing neurons (red). A break in the Y-axis was inserted between 5 and 60 µm. ). (D) Confocal images of spines in fluorescent neurons containing: SCR-132 (black) or miR-132 (red). (E) Bar graphs of the normalized spine density. N = 3 mice for each condition. (F) Representative traces of GABAergic postsynaptic synaptic currents (PSCs) in SCR-132- and miR-132-containing neurons. (G and H) Bar graphs of the frequency (E) and amplitude (F) of GABAergic PSCs in SCR-132- and miR-132-containing neurons (n = 10 black and 15 red, respectively). Scale bar: 100 pA/500 ms in F.
Figure 5. miR-132 overexpression at synaptic integration promotes long-term neuronal survival.
(A) qRT-PCR of miR-132 fold-change normalized to control RNA U6 from the ipsilateral bulbs containing pSico132-expressing neurons (red) and from the contralateral bulbs (black) at 5 weeks post-tamoxifen (wpt) injections given 2 wpe (N = 5 mice each). (B and C) Plots of the summed dendrite length (B) and bar graphs of the total dendritic length (C) of pSicoSCR (n = 47) and pSico132-containing neurons (n = 51) at 5 wpt injections given at 7 dpe (i.e. 6 wpe). (D) Bar graphs of the frequency of GABAergic PSCs in pSicoSCR- and pSico132-containing neurons at 5 wpt (n = 13 black and 15 red, respectively). (G) Sample images illustrating the density of pSicoSCR and pSico132 neurons in OB coronal sections. (H and I) Bar graphs of absolute (H) and normalized (I) RFP+ (i.e. pSicoSCR, black and pSico132, red) neuron density in the GCL (N = 8 and 9 mice, 3–4 images per mouse, respectively). Scale bar: 100 µm.
Similar articles
- MiR-124 Promotes Newborn Olfactory Bulb Neuron Dendritic Morphogenesis and Spine Density.
Li G, Ling S. Li G, et al. J Mol Neurosci. 2017 Feb;61(2):159-168. doi: 10.1007/s12031-016-0873-x. Epub 2016 Dec 6. J Mol Neurosci. 2017. PMID: 27924451 - CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis.
Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. Herold S, et al. Mol Cell Neurosci. 2011 Jan;46(1):79-88. doi: 10.1016/j.mcn.2010.08.008. Epub 2010 Aug 27. Mol Cell Neurosci. 2011. PMID: 20801218 - Early formation of GABAergic synapses governs the development of adult-born neurons in the olfactory bulb.
Pallotto M, Nissant A, Fritschy JM, Rudolph U, Sassoè-Pognetto M, Panzanelli P, Lledo PM. Pallotto M, et al. J Neurosci. 2012 Jun 27;32(26):9103-15. doi: 10.1523/JNEUROSCI.0214-12.2012. J Neurosci. 2012. PMID: 22745509 Free PMC article. - Integration and maturation of newborn neurons in the adult olfactory bulb--from synapses to function.
Nissant A, Pallotto M. Nissant A, et al. Eur J Neurosci. 2011 Mar;33(6):1069-77. doi: 10.1111/j.1460-9568.2011.07605.x. Eur J Neurosci. 2011. PMID: 21395850 Review. - Olfactory bulb neurogenesis depending on signaling in the subventricular zone.
Chen Y, Ren P, He X, Yan F, Gu R, Bai J, Zhang X. Chen Y, et al. Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review.
Cited by
- An emerging role for microRNAs in sexually dimorphic neurobiological systems.
Pak TR, Rao YS, Prins SA, Mott NN. Pak TR, et al. Pflugers Arch. 2013 May;465(5):655-67. doi: 10.1007/s00424-013-1227-y. Epub 2013 Feb 9. Pflugers Arch. 2013. PMID: 23397171 Free PMC article. Review. - Hypoxia-inducible factor 1a is a Tsc1-regulated survival factor in newborn neurons in tuberous sclerosis complex.
Feliciano DM, Zhang S, Quon JL, Bordey A. Feliciano DM, et al. Hum Mol Genet. 2013 May 1;22(9):1725-34. doi: 10.1093/hmg/ddt018. Epub 2013 Jan 24. Hum Mol Genet. 2013. PMID: 23349360 Free PMC article. - A role for miR-132 in learned safety.
Ronovsky M, Zambon A, Cicvaric A, Boehm V, Hoesel B, Moser BA, Yang J, Schmid JA, Haubensak WE, Monje FJ, Pollak DD. Ronovsky M, et al. Sci Rep. 2019 Jan 24;9(1):528. doi: 10.1038/s41598-018-37054-z. Sci Rep. 2019. PMID: 30679653 Free PMC article. - Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification.
Feliciano DM, Zhang S, Nasrallah CM, Lisgo SN, Bordey A. Feliciano DM, et al. PLoS One. 2014 Feb 12;9(2):e88810. doi: 10.1371/journal.pone.0088810. eCollection 2014. PLoS One. 2014. PMID: 24533152 Free PMC article. - Noncoding RNAs and neurobehavioral mechanisms in psychiatric disease.
Kocerha J, Dwivedi Y, Brennand KJ. Kocerha J, et al. Mol Psychiatry. 2015 Jun;20(6):677-684. doi: 10.1038/mp.2015.30. Epub 2015 Mar 31. Mol Psychiatry. 2015. PMID: 25824307 Free PMC article. Review.
References
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. - PubMed
- Pignatelli A, Belluzzi O. Neurogenesis in the Adult Olfactory Bulb. In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton, Fl: 2010. - PubMed
- Bordey A. Adult Neurogenesis: Basic Concepts of Signaling. Cell Cycle. 2006;5:722–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC007681/DC/NIDCD NIH HHS/United States
- P30 HD003352/HD/NICHD NIH HHS/United States
- G0902418/MRC_/Medical Research Council/United Kingdom
- R01 NS069695/NS/NINDS NIH HHS/United States
- P30 NS-052519/NS/NINDS NIH HHS/United States
- R01 MH078972/MH/NIMH NIH HHS/United States
- P30 NS052519/NS/NINDS NIH HHS/United States
- R01 MH080434/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous