The ATPase activity of the P-glycoprotein drug pump is highly activated when the N-terminal and central regions of the nucleotide-binding domains are linked closely together - PubMed (original) (raw)
The ATPase activity of the P-glycoprotein drug pump is highly activated when the N-terminal and central regions of the nucleotide-binding domains are linked closely together
Tip W Loo et al. J Biol Chem. 2012.
Abstract
The P-glycoprotein (P-gp, ABCB1) drug pump protects us from toxic compounds and confers multidrug resistance. Each of the homologous halves of P-gp is composed of a transmembrane domain (TMD) with 6 TM segments followed by a nucleotide-binding domain (NBD). The predicted drug- and ATP-binding sites reside at the interface between the TMDs and NBDs, respectively. Crystal structures and EM projection images suggest that the two halves of P-gp are separated by a central cavity that closes upon binding of nucleotide. Binding of drug substrates may induce further structural rearrangements because they stimulate ATPase activity. Here, we used disulfide cross-linking with short (8 Å) or long (22 Å) cross-linkers to identify domain-domain interactions that activate ATPase activity. It was found that cross-linking of cysteines that lie close to the LSGGQ (P517C) and Walker A (I1050C) sites of NBD1 and NBD2, respectively, as well as the cytoplasmic extensions of TM segments 3 (D177C or L175C) and 9 (N820C) with a short cross-linker activated ATPase activity over 10-fold. A pyrylium compound that inhibits ATPase activity blocked cross-linking at these sites. Cross-linking between the NBDs was not inhibited by tariquidar, a drug transport inhibitor that stimulates P-gp ATPase activity but is not transported. Cross-linking between extracellular cysteines (T333C/L975C) predicted to lock P-gp into a conformation that prevents close NBD association inhibited ATPase activity. The results suggest that trapping P-gp in a conformation in which the NBDs are closely associated likely mimics the structural rearrangements caused by binding of drug substrates that stimulate ATPase activity.
Figures
FIGURE 1.
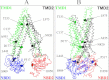
Schematic models of P-gp in the inward- and outward-facing conformations. The predicted models of human P-gp in the open (A) (33) and closed (B) (34) conformations are shown. The branched lines on the extracellular loop connecting TM1 and TM2 represent the glycosylated sites. The colors highlight the TMD1 (green), NBD1 (blue), TMD2 (black), and NBD2 (red) domains. The location of residues that were mutated to cysteine to test for the effect of cross-linking between NBD1 and NBD2 (P517C/I1050C), NBD1 and TMD2 (L443C/S909C), ICL1 and ICL3 (D177C/N820C), TM segments 2 and 11 (C137/A935C) or 6 and 12 (T333C/L975C) are shown.
FIGURE 2.
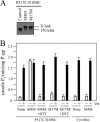
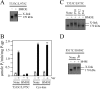
Cross-linking of the NBDs with M4M highly stimulates ATPase activity. A, membranes prepared from HEK 293 cells expressing the NBD1/NBD2 mutant P517C/I1050C were treated without (Control) or with cross-linkers M4M or M17M. The reactions were stopped by addition of SDS sample buffer containing no thiol-reducing agent and samples subjected to immunoblot analysis. The positions of the cross-linked (X-link) and mature (170 kDa) P-gps are indicated. B, membranes expressing mutant P517C/I1050C or Cys-less P-gp were treated with or without (None) cross-linker and histidine-tagged P-gp isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid and ATPase activities were measured in the absence (−) or presence (+) of 0.3 m
m
verapamil. In some cases the samples were treated with 10 m
m
dithiothreitol (+DTT) before assay. Each value is the mean ± S.D. (n = 3).
FIGURE 3.
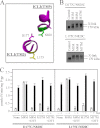
Cross-linking of ICL1 and ICL3 with M4M highly stimulates ATPase activity. A, model showing predicted orientations of Leu175, Asp177, and Asn820 in the closed conformation. B, membranes prepared from HEK 293 cells expressing the ICL1/ICL3 mutants D177C/N820C or L175C/N820C were treated with cross-linkers M1M, M4M, or M17M or no cross-linker (Control). The reactions were stopped by addition of SDS sample buffer containing no thiol-reducing agent and samples subjected to immunoblot analysis. The positions of the cross-linked (X-link) and mature (170 kDa) P-gps are indicated. C, membranes expressing mutants D177C/N820C or L175C/N820C were treated with or without (None) cross-linker and histidine-tagged P-gp isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid and ATPase activities were measured in the absence (−) or presence (+) of 0.3 m
m
verapamil. In some cases the samples were treated with 10 m
m
dithiothreitol (+DTT) before assay. Each value is the mean ± S.D. (n = 3).
FIGURE 4.
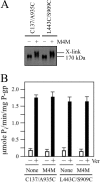
Cross-linking between NBD1 and TMD2 or TM segments 2 and 11 with M4M does not activate or inhibit ATPase activity. A, membranes prepared from HEK 293 cells expressing the NBD1/TMD2 mutant L443C/S909C or the TM2/TM11 mutant C137/A935C were treated with (+) or without (−) the M4M cross-linker. The reactions were stopped by addition of SDS sample buffer containing no thiol-reducing agent and samples subjected to immunoblot analysis. The positions of the cross-linked (X-link) and mature (170 kDa) P-gps are indicated. B, membranes expressing mutants L443C/S909C or C137/A935C were treated with or without (None) M4M cross-linker and histidine-tagged P-gp isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid and ATPase activities were measured in the absence (−) or presence (+) of 0.3 m
m
verapamil. Each value is the mean ± S.D. (n = 3).
FIGURE 5.
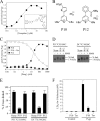
Pyrylium compound P10 inhibits NBD cross-linking, ATPase activity and P-gp-mediated drug resistance. A, ATPase activity of isolated histidine-tagged Cys-less P-gp was determined in the presence of various concentrations of tariquidar (structure shown in inset). B, structures of pyrylium compounds P10 and P12. C, Cys-less P-gp ATPase activity was determined in the presence of various concentrations of P10 or P12. Inhibition of verapamil-stimulated ATPase activity was performed in the presence of 0.1 m
m
verapamil and increasing concentrations of P10. D, membranes prepared from HEK 293 cells expressing mutants P517C(NBD1)/I1050C(NBD2) or D177C(ICL1)/N820C(ICL3) were pretreated in the absence (None) or presence of 0.1 m
m
P10 or P12. The membranes were then treated with (+) or without (−) 0.05 m
m
M4M cross-linker and samples subjected to immunoblot analysis. The positions of cross-linked (X-link) and mature (170 kDa) forms of P-gp are indicated. E, percent of cross-linked product relative to total P-gp (cross-linked plus 170 kDa P-gp) was determined. Each value is the mean ± S.D. (n = 3). F, BHK cells (Control) or BHK cells expressing wild-type P-gp were incubated in the presence of various levels of colchicine in the presence (+) or absence (−) of 5 μ
m
P10 or 100 n
m
tariquidar and the concentration of colchicine required to reduce cell viability by 50% (LD50) was determined. The results represent the average LD50 obtained from analysis of three independent assays ± S.D.
FIGURE 6.
Cross-linking of cysteines on the extracellular surface inhibits ATPase activity. A, cells expressing mutant T333C(TM6)/L975C(TM12) were treated with (+) or without (−) 0.5 m
m
BMOE. Samples were subjected to immunoblot analysis. B, cells expressing Cys-less P-gp or mutant T333C(TM6)/L975C(TM12) were treated with 0.5 m
m
BMOE. P-gp was then isolated by nickel-chelate chromatography and ATPase activity determined in the presence (+) or absence (−) of 0.3 m
m
verapamil. Each value is the mean ± S.D. (n = 3). C, cells expressing mutant T333C/L975C were pretreated with 0.1 m
m
P10, 0.1 m
m
P12, 0.03 m
m
tariquidar (Tar), or no compound (None) and then incubated in the absence (−) or presence (+) of 0.5 m
m
BMOE. Samples were subjected to immunoblot analysis. D, membranes prepared from cells expressing mutant P517C/I1050C were pretreated without (None) or with 0.03 m
m
tariquidar (Tar) followed by incubation in the absence (−) or presence (+) of 0.05 m
m
M4M cross-linker. Samples were then subjected to immunoblot analysis. The positions of mature (170 kDa) and cross-linked (X-link) P-gps are indicated.
FIGURE 7.
Models based on open and closed crystal structures showing relative locations of NBD1 LSGGQ and NBD2 Walker A sites. A, model of P-gp in the closed conformation showing the N-terminal LSGGQ sequence (residues 531–535) to be in close proximity to the C-terminal Walker A sequence (residues 1070–1077) if the P517C/I1050C or D177C/N820C pairs of cysteines were cross-linked with M4M. B, P-gp in the open conformation in which these residues are far apart.
FIGURE 8.
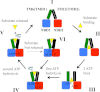
Models of P-gp catalytic cycle and constitutive activation of ATPase activity. For clarity, only TM6 and TM12 of TMD1 and TMD2 are shown since we previously showed that hydrolysis of ATP or binding of drug substrate can promote rotation or conformational changes with these TM segments (19, 51). The cycle starts with P-gp in an open conformation (I). Although binding of drug substrate is shown as the first step for discussion, binding of drug substrate and ATP can bind in a random order. Binding of drug substrate can alter packing of the TM segments by an induced-fit mechanism (II) (19). Binding of ATP has been shown to cause repacking of the TM segments (III) (66) and cause a decrease in affinity for some drug substrates (67). Binding of ATP also promotes formation of the nucleotide-sandwich conformation and an outward-facing conformation (IV). ATP hydrolysis at one site causes rotation or movement of the TM segments (51). If drug is released, then hydrolysis of the second ATP would reset P-gp to a resting state. If the drug substrate is not released, then hydrolysis of an ATP molecule at the second site causes additional conformational changes to promote drug release (V). If the substrate is not released (example would be covalent attachment of a thiol-reactive drug substrate to a cysteine in the TM segments) (VI) or the NBDs are held in close proximity by cross-linking then P-gp will continue to hydrolyze ATP at a high rate.
Similar articles
- Identification of the distance between the homologous halves of P-glycoprotein that triggers the high/low ATPase activity switch.
Loo TW, Clarke DM. Loo TW, et al. J Biol Chem. 2014 Mar 21;289(12):8484-92. doi: 10.1074/jbc.M114.552075. Epub 2014 Feb 12. J Biol Chem. 2014. PMID: 24523403 Free PMC article. - Drug binding in human P-glycoprotein causes conformational changes in both nucleotide-binding domains.
Loo TW, Bartlett MC, Clarke DM. Loo TW, et al. J Biol Chem. 2003 Jan 17;278(3):1575-8. doi: 10.1074/jbc.M211307200. Epub 2002 Nov 5. J Biol Chem. 2003. PMID: 12421806 - P-glycoprotein-mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationships.
Di Pietro A, Dayan G, Conseil G, Steinfels E, Krell T, Trompier D, Baubichon-Cortay H, Jault J. Di Pietro A, et al. Braz J Med Biol Res. 1999 Aug;32(8):925-39. doi: 10.1590/s0100-879x1999000800001. Braz J Med Biol Res. 1999. PMID: 10454753 Review. - Structural and functional aspects of P-glycoprotein and its inhibitors.
Mollazadeh S, Sahebkar A, Hadizadeh F, Behravan J, Arabzadeh S. Mollazadeh S, et al. Life Sci. 2018 Dec 1;214:118-123. doi: 10.1016/j.lfs.2018.10.048. Epub 2018 Oct 27. Life Sci. 2018. PMID: 30449449 Review.
Cited by
- Transport of Alzheimer's associated amyloid-β catalyzed by P-glycoprotein.
McCormick JW, McCormick L, Chen G, Vogel PD, Wise JG. McCormick JW, et al. PLoS One. 2021 Apr 26;16(4):e0250371. doi: 10.1371/journal.pone.0250371. eCollection 2021. PLoS One. 2021. PMID: 33901197 Free PMC article. - Cryo-EM Analysis of the Conformational Landscape of Human P-glycoprotein (ABCB1) During its Catalytic Cycle.
Frank GA, Shukla S, Rao P, Borgnia MJ, Bartesaghi A, Merk A, Mobin A, Esser L, Earl LA, Gottesman MM, Xia D, Ambudkar SV, Subramaniam S. Frank GA, et al. Mol Pharmacol. 2016 Jul;90(1):35-41. doi: 10.1124/mol.116.104190. Epub 2016 May 11. Mol Pharmacol. 2016. PMID: 27190212 Free PMC article. - A Conformationally Gated Model of Methadone and Loperamide Transport by P-Glycoprotein.
Gibbs ME, Wilt LA, Ledwitch KV, Roberts AG. Gibbs ME, et al. J Pharm Sci. 2018 Jul;107(7):1937-1947. doi: 10.1016/j.xphs.2018.02.019. Epub 2018 Feb 28. J Pharm Sci. 2018. PMID: 29499278 Free PMC article. - Cooperativity between verapamil and ATP bound to the efflux transporter P-glycoprotein.
Ledwitch KV, Gibbs ME, Barnes RW, Roberts AG. Ledwitch KV, et al. Biochem Pharmacol. 2016 Oct 15;118:96-108. doi: 10.1016/j.bcp.2016.08.013. Epub 2016 Aug 13. Biochem Pharmacol. 2016. PMID: 27531061 Free PMC article.
References
- Juliano R. L., Ling V. (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455, 152–162 - PubMed
- Sharom F. J. (2006) Shedding light on drug transport: structure and function of the P-glycoprotein multidrug transporter (ABCB1). Biochem. Cell Biol. 84, 979–992 - PubMed
- Ambudkar S. V., Dey S., Hrycyna C. A., Ramachandra M., Pastan I., Gottesman M. M. (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39, 361–398 - PubMed
- Eckford P. D., Sharom F. J. (2009) ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 109, 2989–3011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous