Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation - PubMed (original) (raw)
Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation
Xiao-Yu Zheng et al. Cell Host Microbe. 2012.
Abstract
Phytopathogens can manipulate plant hormone signaling to access nutrients and counteract defense responses. Pseudomonas syringae produces coronatine, a toxin that mimics the plant hormone jasmonic acid isoleucine and promotes opening of stomata for bacterial entry, bacterial growth in the apoplast, systemic susceptibility, and disease symptoms. We examined the mechanisms underlying coronatine-mediated virulence and show that coronatine activates three homologous NAC transcription factor (TF) genes, ANAC019, ANAC055, and ANAC072, through direct activity of the TF, MYC2. Genetic characterization of NAC TF mutants demonstrates that these TFs mediate coronatine-induced stomatal reopening and bacterial propagation in both local and systemic tissues by inhibiting the accumulation of the key plant immune signal salicylic acid (SA). These NAC TFs exert this inhibitory effect by repressing ICS1 and activating BSMT1, genes involved in SA biosynthesis and metabolism, respectively. Thus, a signaling cascade by which coronatine confers its multiple virulence activities has been elucidated.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1
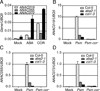
Psm ES4326 induces the expression of ANAC019, ANAC055 and ANAC072 through the ABA- and JA-signaling pathways as well as COR. (A) ANAC019, ANAC055 and ANAC072 transcripts were measured in wild type (Col-0) treated with either ABA, COR or mock. (B-D) Col-0 plants, the ABA-deficient mutant aba2-1 and the JA-signaling mutant coi1-3 were pressure-infiltrated with the mock solution (Mock), Psm ES4326 (Psm) or Psm ES4326 cor− (Psm cor−) and samples were collected 24 hours later. Error bars represent the standard deviation of three technical replicates. Each experiment was repeated at least three times with similar results. See also Figure S1.
Figure 2
MYC2 induces the expression of ANAC019, ANAC055 and ANAC072 by directly interacting with their promoters. (A-C) Transcript levels of ANAC019, ANAC055 and ANAC072 were measured in Col-0 and myc2 in response to mock, Psm ES4326 or Psm ES4326 cor−. Error bars represent standard deviation of three technical replicates. (D-F) 35S:MYC2-GFP transgenic plants were used for ChIP experiments. Long horizontal lines represent the promoters. Ticks above the lines represent putative MYC2 binding sites. Short horizontal lines show the regions where different qPCR primers amplify. Error bars represent standard error of three technical replicates. Each experiment was repeated at least three times with similar results. See also Figure S2 and Table S1.
Figure 3
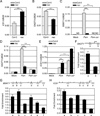
ANAC019, ANAC055 and ANAC072 are involved in COR-triggered virulence in stomata, apoplast and systemic tissue. (A) Stomata aperture from leaf peels of Col-0, the nac triple mutant and the coi1-3 mutant treated with mock, COR, ABA or combination of COR and ABA for 1 hour. (B–C) Stomatal closure response in leaf peels of Col-0, the nac triple mutant and the coi1-3 mutant after 1-hour incubation (B) or 4-hour incubation (C) with either mock or Pst DC3000. Error bars represent standard error. Each experiment was repeated at least twice with similar results. Student’s t test was used to compare means between mock and specific treatment of the same genotype. (D) Col-0, the nac triple mutant and the coi1-3 mutant were dip-inoculated with Psm ES4326. Pictures of the disease symptoms were taken at 3 dpi and bacterial growth was measured at 4 hpi, 1 and 3 dpi. Student’s t test was used to compare the log-transformed data of different genotypes at the same time point. (E) Col-0, the single, double mutants of ANAC019, ANAC055 and ANAC072 and the nac triple mutant were pressure-infiltrated with Psm ES4326. Photographs of the disease symptoms were taken 3 dpi. Bacterial growth was measured at the same time. Bonferroni's multiple comparison test was preformed to compare the log-transformed data of each mutant to that of Col-0. (F) Col-0 and the nac triple mutant were pressure-infiltrated with Psm ES4326 or Psm ES4326 cor− and assayed 3 dpi. (G) Psm ES4326 growth in systemic leaves with pre-inoculation with Psm ES4326 or Psm ES4326 cor−. Error bars represent 95% confidence intervals. Experiments were repeated for at least twice with similar results. ns, no significant difference. See also Figure S3.
Figure 4
COR suppresses SA accumulation through ANAC019, ANAC055 and ANAC072 to promote virulence. Col-0 and the nac triple mutant were pressure-infiltrated with Psm ES4326 or Psm ES4326 cor−. Samples were collected at specified time points to measure the levels of SA (A), SAG (B) and MeSA (C). Error bars represent standard deviation of three replicates. Student’s t test was used to compare two means at the same time point. (D) Psm ES4326 growth was measured in Col-0, the nac triple mutant, and the nac triple mutant overexpressing BSMT1 (BSMT1-OE/nac) as described in Figure 3E. Error bars represent 95% confidence intervals. Experiments were repeated at least twice with similar results. See also Figure S4 and Table S2.
Figure 5
ANAC019, ANAC055 and ANAC072 differentially regulate ICS1, BSMT1 and SAGT1 through direct interaction with their promoters. Basal transcript levels of ICS1 (A) and SAGT1 (B) were measured in three-week-old leaves. Col-0 and the nac triple mutant were pressure-infiltrated with 10 mM MgCl2, Psm ES4326 or Psm ES4326 cor− and were collected after 24 hours to measure the expression of BSMT1 (C), SAGT1 (D), and ICS1 (E). For ICS1, a higher dose of pathogen (OD600nm = 0.02) was used. ND: no detectable signal. Error bars represent standard deviation of three technical replicates. (F-H) ChIP experiments were performed using the Psm ES4326-infected 35S:ANAC019-GFP transgenic plants. Long horizontal lines represent the promoters of SAGT1 (F), BSMT1 (G) and ICS1 (H). Ticks above the lines represent NAC core-binding sites. Short horizontal lines show the regions where different qPCR primers amplify. Error bars represent standard error. Experiments were repeated three times with similar results. See also Figure S5 and Table S3.
Figure 6
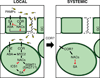
A model of the signaling cascade mediating the virulence effects of COR. In stomata, PAMPs from the pathogen trigger stomatal closure through SA and ABA. COR produced by Pseudomonas inhibits this effect and reopens the stomata through ANAC019, ANAC055 and ANAC072 (NACs). In apoplast, COR induces the ANAC019, ANAC055 and ANAC072 by the MYC2 TF. Type III effectors (T3E) might also induce the NACs through ABA signaling. These TFs then repress ICS1 and induce BSMT1 and possibly SAGT1 to inhibit SA accumulation and promote bacterial virulence. In systemic tissue, ANAC019, ANAC055 and ANAC072 are induced possibly by translocated COR or a COR-generated signal. GC, guard cell.
Similar articles
- The coronatine toxin of Pseudomonas syringae is a multifunctional suppressor of Arabidopsis defense.
Geng X, Cheng J, Gangadharan A, Mackey D. Geng X, et al. Plant Cell. 2012 Nov;24(11):4763-74. doi: 10.1105/tpc.112.105312. Epub 2012 Nov 30. Plant Cell. 2012. PMID: 23204405 Free PMC article. - A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000.
Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, He SY. Zeng W, et al. PLoS Pathog. 2011 Oct;7(10):e1002291. doi: 10.1371/journal.ppat.1002291. Epub 2011 Oct 6. PLoS Pathog. 2011. PMID: 21998587 Free PMC article. - Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack.
Du M, Zhai Q, Deng L, Li S, Li H, Yan L, Huang Z, Wang B, Jiang H, Huang T, Li CB, Wei J, Kang L, Li J, Li C. Du M, et al. Plant Cell. 2014 Jul;26(7):3167-84. doi: 10.1105/tpc.114.128272. Epub 2014 Jul 8. Plant Cell. 2014. PMID: 25005917 Free PMC article. - The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae.
Geng X, Jin L, Shimada M, Kim MG, Mackey D. Geng X, et al. Planta. 2014 Dec;240(6):1149-65. doi: 10.1007/s00425-014-2151-x. Epub 2014 Aug 26. Planta. 2014. PMID: 25156488 Free PMC article. Review. - Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants.
Xin XF, He SY. Xin XF, et al. Annu Rev Phytopathol. 2013;51:473-98. doi: 10.1146/annurev-phyto-082712-102321. Epub 2013 May 31. Annu Rev Phytopathol. 2013. PMID: 23725467 Review.
Cited by
- Two serine residues in Pseudomonas syringae effector HopZ1a are required for acetyltransferase activity and association with the host co-factor.
Ma KW, Jiang S, Hawara E, Lee D, Pan S, Coaker G, Song J, Ma W. Ma KW, et al. New Phytol. 2015 Dec;208(4):1157-68. doi: 10.1111/nph.13528. Epub 2015 Jun 23. New Phytol. 2015. PMID: 26103463 Free PMC article. - A NAC triad modulates plant immunity by negatively regulating N-hydroxy pipecolic acid biosynthesis.
Cai J, Panda S, Kazachkova Y, Amzallag E, Li Z, Meir S, Rogachev I, Aharoni A. Cai J, et al. Nat Commun. 2024 Aug 22;15(1):7212. doi: 10.1038/s41467-024-51515-2. Nat Commun. 2024. PMID: 39174537 Free PMC article. - Coronatine inhibits stomatal closure and delays hypersensitive response cell death induced by nonhost bacterial pathogens.
Lee S, Ishiga Y, Clermont K, Mysore KS. Lee S, et al. PeerJ. 2013 Feb 12;1:e34. doi: 10.7717/peerj.34. Print 2013. PeerJ. 2013. PMID: 23638370 Free PMC article. - Pseudomonas syringae pv. syringae uses proteasome inhibitor syringolin A to colonize from wound infection sites.
Misas-Villamil JC, Kolodziejek I, Crabill E, Kaschani F, Niessen S, Shindo T, Kaiser M, Alfano JR, van der Hoorn RA. Misas-Villamil JC, et al. PLoS Pathog. 2013 Mar;9(3):e1003281. doi: 10.1371/journal.ppat.1003281. Epub 2013 Mar 28. PLoS Pathog. 2013. PMID: 23555272 Free PMC article. - Salt-Related MYB1 Coordinates Abscisic Acid Biosynthesis and Signaling during Salt Stress in Arabidopsis.
Wang T, Tohge T, Ivakov A, Mueller-Roeber B, Fernie AR, Mutwil M, Schippers JH, Persson S. Wang T, et al. Plant Physiol. 2015 Oct;169(2):1027-41. doi: 10.1104/pp.15.00962. Epub 2015 Aug 4. Plant Physiol. 2015. PMID: 26243618 Free PMC article.
References
- Block A, Schmelz E, Jones JB, Klee HJ. Coronatine and salicylic acid: the battle between Arabidopsis and Pseudomonas for phytohormone control. Mol Plant Pathol. 2005;6:79–83. - PubMed
- Bostock RM. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annual review of phytopathology. 2005;43:545–580. - PubMed
- Brooks DM, Bender CL, Kunkel BN. The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol. 2005;6:629–639. - PubMed
- Brooks DM, Hernandez-Guzman G, Kloek AP, Alarcon-Chaidez F, Sreedharan A, Rangaswamy V, Penaloza-Vazquez A, Bender CL, Kunkel BN. Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact. 2004;17:162–174. - PubMed
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI068718/AI/NIAID NIH HHS/United States
- R01 GM069594/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01AI068718/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous