'Ribozoomin'--translation initiation from the perspective of the ribosome-bound eukaryotic initiation factors (eIFs) - PubMed (original) (raw)
Review
'Ribozoomin'--translation initiation from the perspective of the ribosome-bound eukaryotic initiation factors (eIFs)
Leos Shivaya Valásek. Curr Protein Pept Sci. 2012 Jun.
Free PMC article
Abstract
Protein synthesis is a fundamental biological mechanism bringing the DNA-encoded genetic information into life by its translation into molecular effectors - proteins. The initiation phase of translation is one of the key points of gene regulation in eukaryotes, playing a role in processes from neuronal function to development. Indeed, the importance of the study of protein synthesis is increasing with the growing list of genetic diseases caused by mutations that affect mRNA translation. To grasp how this regulation is achieved or altered in the latter case, we must first understand the molecular details of all underlying processes of the translational cycle with the main focus put on its initiation. In this review I discuss recent advances in our comprehension of the molecular basis of particular initiation reactions set into the context of how and where individual eIFs bind to the small ribosomal subunit in the pre-initiation complex. I also summarize our current knowledge on how eukaryotic initiation factor eIF3 controls gene expression in the gene-specific manner via reinitiation.
Figures
Fig. (1)
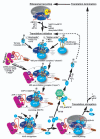
Schematic of the canonical translation pathway in eukaryotes with the ribosomal recycling and initiation phases shown in detail. This figure combines findings from both yeast and mammals and indicates potential differences. The terminating 80S ribosome is split into individual subunits with help of ABCE1/RLI1 and eIFs 1, 1A and 3. How eRFs 1 and 3 are recycled is not properly understood. The former eIFs either remain bound to the 40S subunit or dissociate prior to the initiation phase. In the former case, the Met-tRNAiMet•eIF2•GTP ternary complex (TC) and eIF5 join the existing 40S-eIF1-eIF1A-eIF3 post-recycling complex in a “stochastic” way (i) to form the 43S pre-initiation complex (PIC). In the latter case, the 43S PIC is formed in the “higher order” manner via simultaneous binding of all components of the Multifactor complex (eIFs 1, 3, 5 and the TC) and eIF1A. Upon binding, eIFs1 and 1A induce conformational change that opens the mRNA binding channel of the 40S ribosome for mRNA loading. As a part of this major rearrangement eIF1, if delivered to the ribosome in the MFC, must translocate from eIF3 to the P-site. mRNA is delivered to the 43S PIC in a complex with eIF4F (composed of eIF4A, E and G), eIF4B (and/or eIF4H in mammals) and PABP in an ATP-dependent reaction creating a “landing pad” close to the mRNA’s cap structure that is bound by eIF4E (the interaction between eIF4G and PABP is shown as a dotted line for simplicity). As a result, the 48S PIC is formed and scanning for AUG commences. The actual attachment of mRNA to the ribosome is believed to be mediated via the eIF4G – eIF3 interaction in mammals (dotted line “M”) that seems to be bridged via eIF5 in yeast (dotted line “Y”; this line is not shown in all cartoons for simplicity). During scanning, all secondary structures that could impede the movement of the PIC in the 5' to 3' direction are melted with help of helicase eIF4A and its co-factors eIF4B or eIF4H at the expense of ATP. Also, eIF5 stimulates GTP hydrolysis on eIF2 (GAP activity), however, the resulting Pi is not released until the AUG is located. Upon AUG recognition, eIF1 as a gatekeeper is either ejected from the ribosome or could move back to eIF3 to allow Pi release triggering reciprocal conformational switch to the closed form of the PIC that arrests scanning. eIF5B then promotes subunit joining that kicks out all interface-side-bound eIFs with the exception of eIF1A, and the solvent-side-bound eIF3 and eIF4F (interactions between eIF3 and two “solvent-side” ribosomal proteins RPS0 and RACK1/ASC1, based on [41,59,117], are indicated). GTP hydrolysis on eIF5B stimulated by the GTPase activating center (GAC) of the large subunit triggers coupled release of eIF5B and eIF1A rendering the resulting 80 initiation complex ready to elongate. It is believed that eIF3 and eIF4F can stay 80S-bound for at least a few elongation cycles thanks to their location on the back of the 40S subunit. eIF2•GDP is released in a binary complex with eIF5 that competes with and thus partially inhibits the action of the GEF eIF2B to exchange GDP for GTP on eIF2 (GDI activity). Upon this exchange, eIF2•GTP is ready to form a new TC that can enter the entire cycle all over again. See text for more details. Two “Translational control (TC) points” briefly mentioned in the main text are indicated by yellow arrows and the mechanism of their action by yellow cross lines; the first targets the eIF4E–eIF4G interaction and the other the GTP/GDP exchange on eIF2 by phosphorylating its α subunit.
Fig. (2)
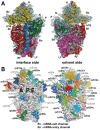
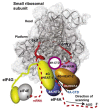
Architecture of the crystal structure of the 40S subunit (adapted from [52]). (A) Interface and solvent-exposed views of the tertiary structure of the 40S showing the 18S rRNA as spheres and colored according to each domain (5' domain, red; central domain, green; 3' major domain, yellow; 3' minor domain, blue; ESs, magenta), and the proteins as gray cartoons (abbreviations: H, head; Be, beak; N, neck; P, platform; Sh, shoulder; Bo, body; RF, right foot; LF, left foot). (B) Ribosomal proteins of the 40S are shown as cartoons in individual colors; rRNA is shown as gray surface. The 40S is shown in the same orientation as in (A).
Fig. (3)
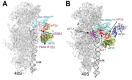
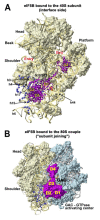
(A - B) Crystal structure of eIF1 in complex with the 40S subunit (adapted from [52]). (A) Eukaryotic initiation factor eIF1 binds to the 18S rRNA phosphate backbone (shown as gray surface) with basic residues (shown in red). The basic loop of eIF1 found in close proximity to the mRNA channel is indicated with an arrow. Genetic experiments implicate residues behind the basic loop, at the end of one of the helices and the penultimate residue in cognate codon recognition (green). (B) T. thermophila eIF1 (red) is located at the top of h44 below the platform. Dashed lines indicate the mRNA path on the 40S subunit. (C - D) Mutual orientations of eIF1A, eIF1, mRNA and Met-tRNAi Met on the 40S subunit (adapted from [75]). Comparison of the positions of eIF1A (shown in blue, red, yellow and green) and eIF1 (violet ribbon) on the 40S subunit. Note that although the modeled position of eIF1A-NTT appears in proximity to eIF1 in this view, the two do not contact each other. (D) View of the mutual orientation of eIF1A, eIF1, mRNA, and Met-tRNAi Met on the 40S subunit, rotated 90° clockwise, compared to panel C. Ribosomal protein S13/RPS18 in the head is not shown as it blocks the view of a portion of eIF1A-CTT.
Fig. (4)
(A) Docking aIF2β on the 40S–aIF2γ–Met-tRNAi Met complex model (adapted from [97]). Helix h1 of aIF2β, which forms the only rigid-body interaction with aIF2γ, is boxed. The aIF2β location corresponding to eIF2β-S264 is shown as purple spheres, and the Met-tRNAi Met residues cleaved by Fe(II)-BABE linked to eIF2ΔC-βS264C are colored purple. (B) Docking of aIF2α, eIF1 and eIF1A on the 40S–aIF2βγ–Met-tRNAi Met complex. Only the eIF1A core structure is shown leaving out both of its terminal tails.
Fig. (5)
(A) A 3D model of eIF3 and its associated eIFs in the MFC (adapted from [38]). ntd, N-terminal domain; ctd, C-terminal domain;hld, HCR1-like domain; rrm, RNA recognition motif; pci, PCI domain; TC, ternary complex. The NMR structure of the interaction between the RRM of human eIF3b (green and light blue) and the N-terminal peptide of human eIF3j (yellow) [17], the NMR structure of the Cterminal RRM of human eIF3g (red and sky-blue) [18], the X-ray structure of the yeast i/TIF34 – b/PRT1-CTD complex [38], and the 3D homology model of the c/NIP1-CTD [59] were used to replace the original schematic representations of the corresponding molecules. (B) Proposed interaction model of human eIF3 based on tandem mass spectometry analysis (adapted from [109]). Subunit organization colored according to signature domains contained within the various subunits. PCI-containing domains (green), MPN domains (red), and RNA recognition motifs (yellow) show direct interactions with the exception of eIF3m and eIF3a. Subunits with no common signature domains are shown in gray. Dashed line is not relevant for the purpose of this review.
Fig. (6)
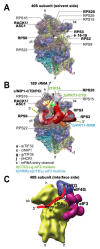
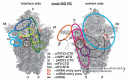
(A – B) A model of two eIF3 modules bound to the opposite termini of the scaffold b/PRT1 subunit situated near the mRNA entry channel of the 40S subunit. (A) The Cryo-EM reconstruction of the 40S subunit is shown from the solvent side with ribosomal RNA represented as tubes. Selected ribosomal proteins are shown as pink cartoons and labeled (adapted from [50]). Positions of RACK1/ASC1, RPS0, 2, 3 and 20 and 18S rRNA helices 16-18 are highlighted in bold. The mRNA entry channel is designated by an asterisk. (B) Hypothetical location of S. cerevisiae eIF3 on the back side of the 40S subunit based on the published interactions between RACK1/ASC1 and c/NIP1- CTD (and potentially also between c/NIP1-PCI and the 18S rRNA segments occurring in the head region) [59]; RPS0 and a/TIF32-NTD [41,117,118]; RPS2 and j/HCR1 [17]; RPS2 and 3 and a/TIF32-CTD [19]; helices 16-18 of 18S rRNA and a/TIF32-CTD [117]; and RPS3 and 20 and g/TIF35 [18] (see text for details). The schematic representations of b/PRT1-CTD and i/TIF34 and of the c/NIP1-CTD were replaced with the X-ray structure [38] or the 3D structural model [59], respectively, as in Fig. 5A. Two eIF3 modules represented by the b/PRT1-CTD–i/TIF34–g/TIF35 and the b/PRT1-RRM–a/TIF32-CTD–j/HCR1 are color-coded in green and blue, respectively. The yellow lines represent mRNA. (C) Cryo-EM docking model of mammalian 40S ribosome bound by eIF3 (violet), eIF4G (blue), eIF1 and mRNA. The A, P, and E sites and mRNA entry and exit sites are indicated; mRNA is depicted as a red line.
Fig. (7)
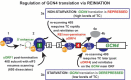
(A) Schematic of the GCN4 mRNA leader showing distribution of all four short uORFs (REI-permissive uORF1 is labeled green; REI-non-permissive uORF4 is labeled red), the predicted structure of the uORF1’s 5’ _cis_-acting sequences (5’ enhancer), 40S- and 80S-bound eIF3, and the description of the mechanism of the GCN4 translation control (adapted from [42]). The 3a and 4a “_GCN4_-expression repressed” steps take places under non-starvation conditions with abundant TC levels, whereas the 3b and 4b “_GCN4_-expression derepressed” steps occur under starvation condition with limited supply of the TC (see text for further details).
Fig. (8)
Hypothetical model for the mechanism of unwinding of mRNA and scanning by the 48S PIC. The small ribosomal subunit is viewed from the solvent face in a gray semi-transparent surface with the rRNA backbone shown as ribbon; the mRNA is illustrated as red solid ribbon. Schematic shows the PIC-associated factors and/or their particular domains including eIF4E and the 4E-BR, the HEAT1, HEAT2 and HEAT3 domains of eIF4G, the CTD and RRM domains of eIF4H, and the NTD and CTD of eIF4A. The direction of scanning of the initiation complex along the mRNA (5' to 3') is indicated by an arrow; eIF4A is shown on the leading (3') side of the scanning complex.
Fig. (9)
(A) The modeled position of eIF5B (purple ribbon) relative to the 30S subunit in the E. coli 70S ribosome crystal structure (adapted from [75]). Blue and orange spheres represent hydroxylradical cleavage positions in 18S rRNA obtained from eIF5B domains II and III, respectively, mapped onto corresponding regions of 16S rRNA. Note that eIF5B domain 4 is in front of, but does not contact the small ribosomal subunit. (B) The modeled position of eIF5B (in surface representation, colored purple) on the E. coli 70S ribosome crystal structure (adapted from [75]).
Fig. (10)
Hypothetical summary model of the structural arrangement of the yeast 48 PIC. Interface and solvent-exposed views of the tertiary structure of the 40S showing the 18S rRNA as spheres and the proteins as gray cartoons (adapted from [52]). Positions of the individual eIFs are schematically depicted as color-coded ovals based on studies referenced throughout this review. Positions with the question marks were not determined experimentally, not even proposed by structural modeling, and thus represent only the author’s best estimate.
Similar articles
- Structural insights into eukaryotic ribosomes and the initiation of translation.
Voigts-Hoffmann F, Klinge S, Ban N. Voigts-Hoffmann F, et al. Curr Opin Struct Biol. 2012 Dec;22(6):768-77. doi: 10.1016/j.sbi.2012.07.010. Epub 2012 Aug 10. Curr Opin Struct Biol. 2012. PMID: 22889726 Review. - Small ribosomal protein RPS0 stimulates translation initiation by mediating 40S-binding of eIF3 via its direct contact with the eIF3a/TIF32 subunit.
Kouba T, Dányi I, Gunišová S, Munzarová V, Vlčková V, Cuchalová L, Neueder A, Milkereit P, Valášek LS. Kouba T, et al. PLoS One. 2012;7(7):e40464. doi: 10.1371/journal.pone.0040464. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792338 Free PMC article. - eIF3: a versatile scaffold for translation initiation complexes.
Hinnebusch AG. Hinnebusch AG. Trends Biochem Sci. 2006 Oct;31(10):553-62. doi: 10.1016/j.tibs.2006.08.005. Epub 2006 Aug 22. Trends Biochem Sci. 2006. PMID: 16920360 Review. - Mechanism of ribosomal subunit joining during eukaryotic translation initiation.
Acker MG, Lorsch JR. Acker MG, et al. Biochem Soc Trans. 2008 Aug;36(Pt 4):653-7. doi: 10.1042/BST0360653. Biochem Soc Trans. 2008. PMID: 18631135 Review. - Translation initiation by cap-dependent ribosome recruitment: Recent insights and open questions.
Shirokikh NE, Preiss T. Shirokikh NE, et al. Wiley Interdiscip Rev RNA. 2018 Jul;9(4):e1473. doi: 10.1002/wrna.1473. Epub 2018 Apr 6. Wiley Interdiscip Rev RNA. 2018. PMID: 29624880 Review.
Cited by
- Differential effects of 40S ribosome recycling factors on reinitiation at regulatory uORFs in GCN4 mRNA are not dictated by their roles in bulk 40S recycling.
Jendruchová K, Gaikwad S, Poncová K, Gunišová S, Valášek LS, Hinnebusch AG. Jendruchová K, et al. Commun Biol. 2024 Sep 4;7(1):1083. doi: 10.1038/s42003-024-06761-x. Commun Biol. 2024. PMID: 39232119 Free PMC article. - SILAC-based quantitative proteomic analysis of human lung cell response to copper oxide nanoparticles.
Edelmann MJ, Shack LA, Naske CD, Walters KB, Nanduri B. Edelmann MJ, et al. PLoS One. 2014 Dec 3;9(12):e114390. doi: 10.1371/journal.pone.0114390. eCollection 2014. PLoS One. 2014. PMID: 25470785 Free PMC article. - In-depth analysis of cis-determinants that either promote or inhibit reinitiation on GCN4 mRNA after translation of its four short uORFs.
Gunišová S, Beznosková P, Mohammad MP, Vlčková V, Valášek LS. Gunišová S, et al. RNA. 2016 Apr;22(4):542-58. doi: 10.1261/rna.055046.115. Epub 2016 Jan 28. RNA. 2016. PMID: 26822200 Free PMC article. - Yeast eIF4A enhances recruitment of mRNAs regardless of their structural complexity.
Yourik P, Aitken CE, Zhou F, Gupta N, Hinnebusch AG, Lorsch JR. Yourik P, et al. Elife. 2017 Nov 30;6:e31476. doi: 10.7554/eLife.31476. Elife. 2017. PMID: 29192585 Free PMC article. - Dynamics of the Pollen Sequestrome Defined by Subcellular Coupled Omics.
Hafidh S, Potěšil D, Müller K, Fíla J, Michailidis C, Herrmannová A, Feciková J, Ischebeck T, Valášek LS, Zdráhal Z, Honys D. Hafidh S, et al. Plant Physiol. 2018 Sep;178(1):258-282. doi: 10.1104/pp.18.00648. Epub 2018 Jul 14. Plant Physiol. 2018. PMID: 30007911 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 090812/WT_/Wellcome Trust/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
- 090812/B/09/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources