Lis1 is an initiation factor for dynein-driven organelle transport - PubMed (original) (raw)
Lis1 is an initiation factor for dynein-driven organelle transport
Martin J Egan et al. J Cell Biol. 2012.
Abstract
The molecular motor cytoplasmic dynein is responsible for most minus-end-directed, microtubule-based transport in eukaryotic cells. It is especially important in neurons, where defects in microtubule-based motility have been linked to neurological diseases. For example, lissencephaly is caused by mutations in the dynein-associated protein Lis1. In this paper, using the long, highly polarized hyphae of the filamentous fungus Aspergillus nidulans, we show that three morphologically and functionally distinct dynein cargos showed transport defects in the genetic absence of Lis1/nudF, raising the possibility that Lis1 is ubiquitously used for dynein-based transport. Surprisingly, both dynein and its cargo moved at normal speeds in the absence of Lis1 but with reduced frequency. Moreover, Lis1, unlike dynein and dynactin, was absent from moving dynein cargos, further suggesting that Lis1 is not required for dynein-based cargo motility once it has commenced. Based on these observations, we propose that Lis1 has a general role in initiating dynein-driven motility.
Figures
Figure 1.
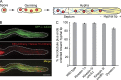
Microtubules are unidirectional from the last nucleus to the hyphal tip. (A) Cartoon depicting the development of a uninucleate A. nidulans spore into a mature multinucleate (in red) hypha, containing unidirectional microtubule arrays (in light and dark blue) from the last nucleus to the growing tip. Microtubule polarity is indicated by plus and minus symbols. (B) Microtubule plus ends are polarized toward the hyphal tip in wild-type hyphae. Microtubules are visualized with GFP–α-tubulin (top), microtubule plus ends with EB1-mCherry, and nuclei with histone H1–mCherry (middle). The bottom image is a merged image of the top and middle images. Dashed lines indicate the outline of the hyphae. Bar, 5 µm. (C) Bar graph showing the percentage of microtubules (±SEM) with plus ends oriented toward the hyphal tip. Only microtubules between the hyphal tip and the most proximal nucleus were analyzed. For each strain, >60 independent hyphae and >700 EB1 comets were analyzed.
Figure 2.
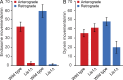
Endosomes, peroxisomes, and nuclei are cargos of dynein and kinesin-3. (A) Bar graphs showing the mean velocities of anterograde- and retrograde-moving endosomes and peroxisomes in wild-type hyphae. Endosomes moved with mean anterograde and retrograde velocities of 2.62 ± 0.83 µm/s (SD) and 2.66 ± 0.77 µm/s, respectively (n = 250). Peroxisomes moved with mean anterograde and retrograde velocities of 1.66 ± 0.77 µm/s (n = 50) and 1.63 ± 0.87 µm/s (n = 48), respectively. (B) Bar graph showing the mean sizes of endosomes and peroxisomes in wild-type hyphae. Organelles were measured along their longest axes. Typical endosomes were round, with a mean diameter of 0.45 ± 0.07 µm (SD), whereas peroxisomes were more elongated, with a mean length of 1.03 ± 0.43 µm (n = 200 for both endosomes and peroxisomes). Endosomes were statistically smaller than peroxisomes (unpaired t test, P < 0.0001). (C and D) Localization of GFP-Rab5/RabA–labeled endosomes, Pex11/PexK-GFP–labeled peroxisomes, and histone H1–mCherry-labeled nuclei after targeted deletion of cargo-transporting motors. Loss of the dynein heavy chain gene resulted in hyphal tip accumulation of both endosomes and peroxisomes and failure of nuclei to distribute properly. In kinesin-1Δ strains, both endosomes and peroxisomes accumulated at the hyphal tip; a slight nuclear misdistribution phenotype was also observed. Deletion of kinesin-3 did not affect nuclei distribution but caused accumulation of endosomes near nuclei and accumulation of peroxisomes in the growing hyphal tip and subapical region. Dashed lines indicate the outline of the hyphae. Bars, 5 µm.
Figure 3.
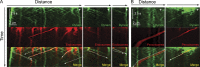
Endosomes and peroxisomes colocalize with moving dynein. (A and B) Kymographs were generated from time-lapse videos. Dynein-3xGFP (top) colocalizes with mCherry-Rab5/RabA–labeled endosomes (A) and mCherry-PTS1–labeled peroxisomes (B; middle) in wild-type hyphae. Bottom images show merged images with white dashed arrows highlighting examples of colocalized events. The locations of microtubule plus ends in the hyphal tip are indicated by white plus signs.
Figure 4.
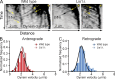
Lis1 is required for the distribution of peroxisomes and endosomes but is not essential for endosome motility in vivo. (A and B) In wild-type hyphae, GFP-Rab5/RabA–labeled endosomes (A) and mCherry-PTS1–labeled peroxisomes (B) are uniformly distributed (left images) and move bidirectionally, as shown in kymographs generated from time-lapse videos (right images). In Lis1Δ hyphae, endosomes (A) and peroxisomes (B) mislocalize to the hyphal tip (left images) and are largely immotile (right images). Dashed lines indicate the outline of the hyphae. The locations of microtubule plus ends in the hyphal tip are indicated by white plus signs. (C) Kymographs of GFP-labeled endosomes in Lis1Δ mutants show examples of Lis1-independent movements of endosomes. (D) Histograms of velocities of anterograde-directed endosomes from wild-type versus Lis1Δ strains. Mean velocities are 2.62 ± 0.83 µm/s (SD) in wild-type hyphae and 2.25 ± 0.85 µm/s in Lis1Δ hyphae (P = 0.0002; n = 250 [wild type]; n = 101 [Lis1Δ]). (E) Histogram of velocities of retrograde-directed endosomes from wild-type versus Lis1Δ strains. Mean velocities are 2.66 ± 0.77 µm/s (SD) in wild-type hyphae and 2.43 ± 0.81 µm/s in Lis1Δ hyphae (P = 0.0169; n = 250 [wild type]; n = 82 [Lis1Δ]).
Figure 5.
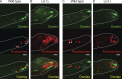
Lis1 is not required for dynein motility. (A) Kymographs of dynein-3xGFP particles reveal movements toward and away from the microtubule plus end in both wild-type and Lis1Δ hyphae. Yellow dashed arrows indicate discrete particle movements toward and away from the hyphal tip. The locations of microtubule plus ends in the hyphal tip are indicated by yellow plus signs. (B) Histograms showing anterograde velocities of dynein-3xGFP particles in wild-type and Lis1Δ hyphae. Mean velocities are 2.09 ± 0.70 µm/s (SD) in wild-type and 2.17 ± 0.52 µm/s in Lis1Δ hyphae (P = 0.197; n = 200 [wild type]; n = 144 [Lis1Δ]). (C) Histograms showing retrograde velocities of dynein-3xGFP particles in wild-type and Lis1Δ hyphae. Mean velocities are 2.38 ± 0.80 µm/s (SD) in wild-type and 2.30 ± 0.75 µm/s in Lis1Δ hyphae (P = 0.420, n = 200 [wild type]; n = 105 [Lis1Δ]).
Figure 6.
The frequencies of organelle and dynein movements are decreased in the absence of Lis1. (A) Bar graph of anterograde and retrograde endosome flux in wild-type and Lis1Δ hyphae. Rates were calculated from the number of endosomes that crossed a line drawn perpendicular to and 10 µm away from the hyphal tip during 3 min of observation. For wild-type hyphae, 127 vesicles moved in the anterograde direction, and 177 vesicles moved in the retrograde direction. For Lis1Δ hyphae, eight vesicles moved in the anterograde direction, and five vesicles moved in the retrograde direction. Error bars represent SD. (B) Bar graph of the rate at which dynein particles enter and leave microtubule plus ends in wild-type and Lis1Δ hyphae. The frequency of anterograde particle movements in Lis1Δ hyphae was not significantly different from that of the wild-type strain (41 ± 4 per minute [SEM] vs. 35 ± 4 per minute, respectively; P = 0.357). The frequency of retrograde dynein particle movements in the absence of Lis1 was significantly lower than that of the wild-type strain (20 ± 6 per minute vs. 48 ± 4 per minute, respectively; P = 0.005; n = 347 [wild type]; n = 142 [Lis1Δ]).
Figure 7.
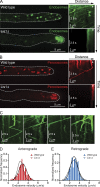
Lis1 is not stably associated with moving dynein cargos. (A) Kymographs of Lis1-GFP (top) and mCherry-Rab5/RabA endosomes (middle) in A. nidulans germlings. Bottom images are merged images of the top and middle images. (B) Bar graph of the percentage of endosomes colocalizing with dynein, Lis1, or dynactin. 93.4 ± 3.0% (SEM) of moving mCherry-Rab5/RabA endosomes colocalized with a dynein-3xGFP particle (n = 110 endosomes). 1.8 ± 1.0% of endosomes colocalized with a Lis1-GFP particle (n = 148 endosomes). 56.4 ± 5.9% of endosomes colocalized with a p25-GFP particle (n = 161 endosomes). (C) Kymographs of p25-GFP (top) and mCherry-Rab5/RabA endosomes (middle) in A. nidulans germlings. Frequent colocalization of p25-GFP and endosomes was observed. Bottom images are merged images of the top and middle images. The locations of microtubule plus ends in the hyphal tip are indicated by white plus signs. White dashed arrows highlight examples of colocalized events.
Figure 8.
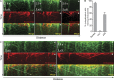
In the absence of Lis1, dynein fails to colocalize with endosomes or peroxisomes at the hyphal tip. (A–D) Micrographs of the extreme tips of wild-type (A and C) and Lis1Δ (B and D) hyphae expressing dynein-3xGFP and mCherry-Rab5/RabA–labeled endosomes (A and B) or mCherry-PTS1–labeled peroxisomes (C and D). In wild-type hyphae, endosomes (A) and peroxisomes (C) localize along the length of microtubules, labeled by dynein-3xGFP, which decorates the microtubule plus ends and localizes to discrete puncta. Dynein-3xGFP colocalized with moving endosomes and peroxisomes (labeled with white asterisks in A and C), as determined by kymograph analysis. In Lis1Δ hyphae, dynein-3xGFP localizes to comets at the microtubule plus ends and to the cytoplasm (B and D, top) but does not colocalize with either endosomes (B, bottom) or peroxisomes (D, bottom) accumulated at the hyphal tip. Dashed lines indicate the outline of the hyphae. Bars, 1 µm.
Figure 9.
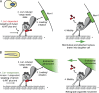
Model of Lis1 function in S. cerevisiae and A. nidulans. (A) In S. cerevisiae, Lis1 (red) is required to recruit dynein (gray) to microtubule plus ends (step 1). Lis1 binding to dynein anchors dynein at the plus end and induces a cargo-ready conformation (step 2). Dynein is then off-loaded to the cortical receptor Num1 (green; step 3). In the absence of Lis1, cortically anchored dynein walks toward the microtubule minus end, biasing movement of the microtubule-attached nucleus into the daughter cell (step 4, green arrow). (B) In A. nidulans hyphae, dynein (gray) is targeted to the microtubule plus end in a Lis1-independent (red), kinesin-1–dependent manner (step 1). Lis1 binding to dynein anchors dynein at the plus end and induces a cargo-ready conformation (step 2). Dynein then binds to endosomes or peroxisomes (green; step 3). In the absence of Lis1, organelle-associated dynein drives retrograde endosome or peroxisome motility (step 4, green arrow).
Similar articles
- Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent.
Zhang J, Li S, Fischer R, Xiang X. Zhang J, et al. Mol Biol Cell. 2003 Apr;14(4):1479-88. doi: 10.1091/mbc.e02-08-0516. Mol Biol Cell. 2003. PMID: 12686603 Free PMC article. - LIS1 regulates cargo-adapter-mediated activation of dynein by overcoming its autoinhibition in vivo.
Qiu R, Zhang J, Xiang X. Qiu R, et al. J Cell Biol. 2019 Nov 4;218(11):3630-3646. doi: 10.1083/jcb.201905178. Epub 2019 Sep 27. J Cell Biol. 2019. PMID: 31562232 Free PMC article. - A microscopy-based screen employing multiplex genome sequencing identifies cargo-specific requirements for dynein velocity.
Tan K, Roberts AJ, Chonofsky M, Egan MJ, Reck-Peterson SL. Tan K, et al. Mol Biol Cell. 2014 Mar;25(5):669-78. doi: 10.1091/mbc.E13-09-0557. Epub 2014 Jan 8. Mol Biol Cell. 2014. PMID: 24403603 Free PMC article. - Cytoplasmic dynein and early endosome transport.
Xiang X, Qiu R, Yao X, Arst HN Jr, Peñalva MA, Zhang J. Xiang X, et al. Cell Mol Life Sci. 2015 Sep;72(17):3267-80. doi: 10.1007/s00018-015-1926-y. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001903 Free PMC article. Review. - [Molecular mechanism of lissencephaly--how LIS1 and NDEL1 regulate cytoplasmic dynein?].
Hirotsune S. Hirotsune S. Brain Nerve. 2008 Apr;60(4):375-81. Brain Nerve. 2008. PMID: 18421979 Review. Japanese.
Cited by
- The journey of the organelle: teamwork and regulation in intracellular transport.
Barlan K, Rossow MJ, Gelfand VI. Barlan K, et al. Curr Opin Cell Biol. 2013 Aug;25(4):483-8. doi: 10.1016/j.ceb.2013.02.018. Epub 2013 Mar 17. Curr Opin Cell Biol. 2013. PMID: 23510681 Free PMC article. Review. - Post-natal treatment by a blood-brain-barrier permeable calpain inhibitor, SNJ1945 rescued defective function in lissencephaly.
Toba S, Tamura Y, Kumamoto K, Yamada M, Takao K, Hattori S, Miyakawa T, Kataoka Y, Azuma M, Hayasaka K, Amamoto M, Tominaga K, Wynshaw-Boris A, Wanibuchi H, Oka Y, Sato M, Kato M, Hirotsune S. Toba S, et al. Sci Rep. 2013;3:1224. doi: 10.1038/srep01224. Epub 2013 Feb 6. Sci Rep. 2013. PMID: 23390575 Free PMC article. - Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells.
Zhou L, Evangelinos M, Wernet V, Eckert AF, Ishitsuka Y, Fischer R, Nienhaus GU, Takeshita N. Zhou L, et al. Sci Adv. 2018 Jan 24;4(1):e1701798. doi: 10.1126/sciadv.1701798. eCollection 2018 Jan. Sci Adv. 2018. PMID: 29387789 Free PMC article. - Dynamic Dissection of Dynein and Kinesin-1 Cooperatively Mediated Intercellular Transport of Porcine Epidemic Diarrhea Coronavirus along Microtubule Using Single Virus Tracking.
Hou W, Kang W, Li Y, Shan Y, Wang S, Liu F. Hou W, et al. Virulence. 2021 Dec;12(1):615-629. doi: 10.1080/21505594.2021.1878748. Virulence. 2021. PMID: 33538234 Free PMC article. - PxdA interacts with the DipA phosphatase to regulate peroxisome hitchhiking on early endosomes.
Salogiannis J, Christensen JR, Songster LD, Aguilar-Maldonado A, Shukla N, Reck-Peterson SL. Salogiannis J, et al. Mol Biol Cell. 2021 Mar 15;32(6):492-503. doi: 10.1091/mbc.E20-08-0559. Epub 2021 Jan 21. Mol Biol Cell. 2021. PMID: 33476181 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous