BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group - PubMed (original) (raw)
Multicenter Study
. 2012 Jul 20;30(21):2654-63.
doi: 10.1200/JCO.2011.39.8545. Epub 2012 Jun 18.
Sian Fereday, Cliff Meldrum, Anna deFazio, Catherine Emmanuel, Joshy George, Alexander Dobrovic, Michael J Birrer, Penelope M Webb, Colin Stewart, Michael Friedlander, Stephen Fox, David Bowtell, Gillian Mitchell
Affiliations
- PMID: 22711857
- PMCID: PMC3413277
- DOI: 10.1200/JCO.2011.39.8545
Multicenter Study
BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group
Kathryn Alsop et al. J Clin Oncol. 2012.
Erratum in
- J Clin Oncol. 2012 Nov 20;30(33):4180
Abstract
Purpose: The frequency of BRCA1 and BRCA2 germ-line mutations in women with ovarian cancer is unclear; reports vary from 3% to 27%. The impact of germ-line mutation on response requires further investigation to understand its impact on treatment planning and clinical trial design.
Patients and methods: Women with nonmucinous ovarian carcinoma (n = 1,001) enrolled onto a population-based, case-control study were screened for point mutations and large deletions in both genes. Survival outcomes and responses to multiple lines of chemotherapy were assessed.
Results: Germ-line mutations were found in 14.1% of patients overall, including 16.6% of serous cancer patients (high-gradeserous, 17.1%); [corrected] 44% had no reported family history of breast orovarian cancer.Patients carrying germ-line mutations had improved rates of progression-free and overall survival. In the relapse setting, patients carrying mutations more frequently responded to both platin- and nonplatin-based regimens than mutation-negative patients, even in patients with early relapse after primary treatment. Mutation-negative patients who responded to multiple cycles of platin-based treatment were more likely to carry somatic BRCA1/2 mutations.
Conclusion: BRCA mutation status has a major influence on survival in ovarian cancer patients and should be an additional stratification factor in clinical trials. Treatment outcomes in BRCA1/2 carriers challenge conventional definitions of platin resistance, and mutation status may be able to contribute to decision making and systemic therapy selection in the relapse setting. Our data, together with the advent of poly(ADP-ribose) polymerase inhibitor trials, supports the recommendation that germ-line BRCA1/2 testing should be offered to all women diagnosed with nonmucinous, ovarian carcinoma, regardless of family history.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
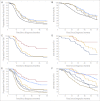
Fig 1.
Kaplan-Meier survival analysis; P values calculated using a log-rank analysis. Estimated (A) progression-free survival (P = .01) and (B) overall survival (P = .01) of women with serous tumors by mutation status: BRCA1 mutation–positive (blue); BRCA2 mutation–positive (gray); BRCA1/2 mutation–positive (combined; gold); wild type (black). Death as a result of disease (n = 371); patients who died as a result of nondisease-related causes were censored (n = 40). Estimated (C) progression-free survival (P < .001) and (D) overall survival (_P_ < .001) of women with serous _BRCA1/2_ mutation–positive tumors by amount of residual disease after primary surgery: nil macroscopic disease (red); ≤ 1 cm (gold); > 1 cm (blue). Death as a result of disease (n = 57); patients who died as a result of nondisease-related causes were censored (n = 4). Estimated (E) progression-free survival (P < .001) and (F) overall survival (_P_ < .001) of women with serous tumors by both mutation status and amount of residual disease: _BRCA1/2_ mutation–positive with nil macroscopic disease (red); _BRCA1/2_ mutation–positive with ≤ 1 cm residual disease (gold); _BRCA1/2_ mutation–positive with > 1 cm macroscopic disease (dark blue); BRCA1/2 wild-type with nil residual disease (light blue); BRCA1/2 wild-type with ≤ 1 cm residual disease (gray); BRCA1/2 wild-type with > 1 cm macroscopic disease (dark gold). Death as a result of disease, n = 348; patients who had died as a result of nondisease-related causes were censored (n = 35).
Fig 2.
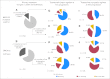
Response to treatment at first progression. Treatment response was based on a 50% decrease in CA-125, maintained for 28 days, as described in the Patients and Methods section. Patients with a sequence variant of unclassified significance were excluded from this analysis. (A) Mutation-positive and (B) mutation-negative patients are divided into those whose disease progressed within 6 months of the end of treatment (dark gray segment) and those whose disease progressed more than 6 months after primary treatment (light gray segment). Response to second line for (C and D) platin-based and (E and F) nonplatin-based treatments in patients with more than 6 months to first progression after primary platinum treatment (n = 333). Response to second line for (G and H) platin-based and (I and J) nonplatin-based treatments in patients whose disease progressed within 6 months of the end of primary platinum treatment (n = 168).
Fig 3.
Time to first progression or death was plotted by mutation position along either the BRCA1 or BRCA2 gene, or by mutation type. Progression is only plotted for patients whose disease progressed by the censoring date; time to death was only plotted for those patients who died as a result of disease-related causes before the censoring date. The line represents the median. Thirty-five of the mutation-positive patients (24.8%) had remained progression free at the time of data censoring for this study. (A) Time to first progression in patients with a BRCA1 mutation who were optimally debulked (nil or ≤ 1 cm residual disease; gold plus symbols) or suboptimally debulked (blue) at time of primary surgery. (B) Time to death in BRCA1 mutation–positive cases who were optimally (gold) or suboptimally (blue) debulked at time of primary surgery. Deaths as a result of disease-related causes (n = 45; plotted); deaths as a result of nondisease-related causes (n = 3; not plotted). (C) Time to first progression in patients with a BRCA2 mutation who were optimally (gold) or suboptimally (blue) debulked at time of primary surgery. (D) Time to death in BRCA2 mutation–positive women who were optimally (gold) or suboptimally (blue) debulked at time of primary surgery. Deaths as a result of disease-related causes (n = 26; plotted); deaths as a result of nondisease-related causes (n = 2; not plotted). (E) Time to first progression by mutation type. A deletion or insertion was considered multiple base if at least two base pairs were involved. (F) Time to death by mutation type. Deaths as a result of disease-related causes (n = 71; plotted); deaths as a result of nondisease-related causes (n = 5; not plotted). A deletion or insertion was considered multiple base if at least two base pairs were involved. HGVS, Human Genome Variation Society.
Similar articles
- Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM, Kristeleit RS, Ma L, Bell-McGuinn KM, Brenton JD, Cragun JM, Oaknin A, Ray-Coquard I, Harrell MI, Mann E, Kaufmann SH, Floquet A, Leary A, Harding TC, Goble S, Maloney L, Isaacson J, Allen AR, Rolfe L, Yelensky R, Raponi M, McNeish IA. Swisher EM, et al. Lancet Oncol. 2017 Jan;18(1):75-87. doi: 10.1016/S1470-2045(16)30559-9. Epub 2016 Nov 29. Lancet Oncol. 2017. PMID: 27908594 Clinical Trial. - Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A'Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB. Fong PC, et al. J Clin Oncol. 2010 May 20;28(15):2512-9. doi: 10.1200/JCO.2009.26.9589. Epub 2010 Apr 20. J Clin Oncol. 2010. PMID: 20406929 Clinical Trial. - Prevalence of Tissue BRCA Gene Mutation in Ovarian, Fallopian Tube, and Primary Peritoneal Cancers: A Multi-Institutional Study.
Lertkhachonsuk AA, Suprasert P, Manchana T, Kittisiam T, Kantathavorn N, Chansoon T, Khunamornpong S, Pohthipornthawat N, Tangjitgamol S, Luasiripanthu T, Teerapakpinyo C, Shuangshot S, Iemwimangsa N, Chantratita W. Lertkhachonsuk AA, et al. Asian Pac J Cancer Prev. 2020 Aug 1;21(8):2381-2388. doi: 10.31557/APJCP.2020.21.8.2381. Asian Pac J Cancer Prev. 2020. PMID: 32856869 Free PMC article. - Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): A comprehensive review.
Patrono MG, Iniesta MD, Malpica A, Lu KH, Fernandez RO, Salvo G, Ramirez PT. Patrono MG, et al. Gynecol Oncol. 2015 Dec;139(3):568-72. doi: 10.1016/j.ygyno.2015.09.018. Epub 2015 Sep 25. Gynecol Oncol. 2015. PMID: 26407480 Review. - Moving toward personalized medicine: treatment-focused genetic testing of women newly diagnosed with ovarian cancer.
Trainer AH, Meiser B, Watts K, Mitchell G, Tucker K, Friedlander M. Trainer AH, et al. Int J Gynecol Cancer. 2010 Jul;20(5):704-16. doi: 10.1111/igc.0b013e3181dbd1a5. Int J Gynecol Cancer. 2010. PMID: 20973257 Review.
Cited by
- Analysis of Founder Mutations in Rare Tumors Associated With Hereditary Breast/Ovarian Cancer Reveals a Novel Association of BRCA2 Mutations with Ampulla of Vater Carcinomas.
Pinto P, Peixoto A, Santos C, Rocha P, Pinto C, Pinheiro M, Leça L, Martins AT, Ferreira V, Bartosch C, Teixeira MR. Pinto P, et al. PLoS One. 2016 Aug 17;11(8):e0161438. doi: 10.1371/journal.pone.0161438. eCollection 2016. PLoS One. 2016. PMID: 27532258 Free PMC article. - PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer.
Hong T, Lei G, Chen X, Li H, Zhang X, Wu N, Zhao Y, Zhang Y, Wang J. Hong T, et al. Redox Biol. 2021 Jun;42:101928. doi: 10.1016/j.redox.2021.101928. Epub 2021 Mar 5. Redox Biol. 2021. PMID: 33722571 Free PMC article. - Landscape of homologous recombination deficiency in gastric cancer and clinical implications for first-line chemotherapy.
Ichikawa H, Aizawa M, Kano Y, Hanyu T, Muneoka Y, Hiroi S, Ueki H, Moro K, Hirose Y, Miura K, Shimada Y, Sakata J, Yabusaki H, Nakagawa S, Kawasaki T, Okuda S, Wakai T. Ichikawa H, et al. Gastric Cancer. 2024 Nov;27(6):1273-1286. doi: 10.1007/s10120-024-01542-1. Epub 2024 Aug 7. Gastric Cancer. 2024. PMID: 39110344 - Stathmin 1 and p16(INK4A) are sensitive adjunct biomarkers for serous tubal intraepithelial carcinoma.
Novak M, Lester J, Karst AM, Parkash V, Hirsch MS, Crum CP, Karlan BY, Drapkin R. Novak M, et al. Gynecol Oncol. 2015 Oct;139(1):104-11. doi: 10.1016/j.ygyno.2015.07.100. Epub 2015 Jul 20. Gynecol Oncol. 2015. PMID: 26206555 Free PMC article. - Untapped Potential of Poly(ADP-Ribose) Polymerase Inhibitors: Lessons Learned From the Real-World Clinical Homologous Recombination Repair Mutation Testing.
Lebedeva A, Veselovsky E, Kavun A, Belova E, Grigoreva T, Orlov P, Subbotovskaya A, Shipunov M, Mashkov O, Bilalov F, Shatalov P, Kaprin A, Shegai P, Diuzhev Z, Migiaev O, Vytnova N, Mileyko V, Ivanov M. Lebedeva A, et al. World J Oncol. 2024 Aug;15(4):562-578. doi: 10.14740/wjon1820. Epub 2024 Jun 11. World J Oncol. 2024. PMID: 38993246 Free PMC article.
References
- Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335:1413–1416. - PubMed
- Sarantaus L, Vahteristo P, Bloom E, et al. BRCA1 and BRCA2 mutations among 233 unselected Finnish ovarian carcinoma patients. Eur J Hum Genet. 2001;9:424–430. - PubMed
- Malander S, Ridderheim M, Måsbäck A, et al. One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: Results of a prospective study in Southern Sweden. Eur J Cancer. 2004;40:422–428. - PubMed
- Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. - PubMed
- Jacobi CE, van Ierland Y, van Asperen CJ, et al. Prediction of BRCA1/2 mutation status in patients with ovarian cancer from a hospital-based cohort. Genet Med. 2007;9:173–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous