Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C - PubMed (original) (raw)
Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C
Helene Labit et al. EMBO J. 2012.
Abstract
The anaphase-promoting complex/cyclosome (APC/C) ubiquitin ligase is tightly regulated to ensure programmed proteolysis in cells. The activity of the APC/C is positively controlled by cyclin-dependent kinase (CDK), but a second level of control must also exist because phosphorylation inactivates Cdc20, a mitotic APC/C co-activator. How Cdc20 is dephosphorylated specifically, when CDK is high, has remained unexplained. Here, we show that phosphatases are crucial to activate the APC/C. Cdc20 is phosphorylated at six conserved residues (S50/T64/T68/T79/S114/S165) by CDK in Xenopus egg extracts. When all the threonine residues are phosphorylated, Cdc20 binding to and activation of the APC/C are inhibited. Their dephosphorylation is regulated depending on the sites and protein phosphatase 2A, active in mitosis, is essential to dephosphorylate the threonine residues and activate the APC/C. Consistently, most of the Cdc20 bound to the APC/C in anaphase evades phosphorylation at T79. Furthermore, we show that the 'activation domain' of Cdc20 associates with the Apc6 and Apc8 core subunits. Our data suggest that dephosphorylation of Cdc20 is required for its loading and activation of the APC/C ubiquitin ligase.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
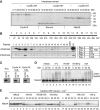
CDK phosphorylation of Cdc20 blocks its activation role. (A) CDK-cyclin B activates interphase APC/C. APC/C-dependent destruction assays were performed in Xenopus interphase extracts and interphase extracts incubated with GST-cyclinBΔ167 (2 μM) for 30 min. 35S-labelled _in vitro_-translated cyclin B (fission yeast Cdc13), securin and Nek2A were used as substrates. Samples were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. (B) Phosphorylation of 19 Cdc20 peptides. (Top panel) A schematic diagram shows the 19 short peptides used as substrates. (Bottom panel) The GST-fused peptides were incubated with Xenopus interphase or anaphase extracts in the presence of [γ-32P]-ATP for 20 min, and then analysed by SDS–PAGE and autoradiography. The table lists the possible phosphorylation sites identified in each peptide. (C) GST-fused Cdc20-N159 or the same fragment with all the CDK sites (S50, T64, T68, T79, S114) mutated to alanine (5A) was incubated with anaphase extracts as well as recombinant CDK-cyclin A or CDK-cyclin B kinases. (D) APC/C was purified from Xenopus mitotic extracts depleted of endogenous Cdc20 and used for in-vitro ubiquitylation assays with buffer (+mock), _in vitro_-translated full-length Cdc20 (+FL), recombinant GST-N159-WT or -5A mock treated (+N159 or 5A) or phosphorylated by recombinant CDK (+N159-p). 35S-labelled _in vitro_-translated Nek2A was used as a substrate. (E) Nek2A destruction was examined in Cdc20-depleted CSF extracts supplemented with N159 or 5A in the presence or absence of OA (2 μM) or phosphorylated by recombinant CDK. Samples were taken at the indicated time points after addition of CaCl2 and analysed by SDS–PAGE and autoradiography.
Figure 2
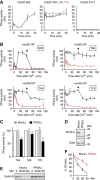
Phosphorylation of the N-terminal domain of Cdc20 blocks the association with the APC/C. (A) APC/C binding assays with the Cdc20 N-terminal domain. Purified N159-WT or -5A was bound to GSH Sepharose and then incubated with Xenopus CSF extract at 23°C for the indicated time. A proteasome inhibitor, MG132 (150 μM) and a CDK inhibitor, roscovitine (500 μM) were added as indicated. The amounts of bound APC/C were analysed by immunoblotting. (B) Quantification of the Apc6 immunoblot in (A). Error bars, s.e.m. from three independent experiments. (C) The amounts of endogenous Cdc20 bound to APC/C in Xenopus extract were examined in the presence of buffer (mock) or recombinant N159-5A. APC/C was purified using anti-Apc3 mAb AF3.1 at the indicated time points after addition of CaCl2 into CSF extract and analysed by immunoblotting. (D) Destruction of 35S-labelled APC/C substrates was examined in the presence of mock, Cdc20-N159-WT or 5A in a cell-free destruction assay. Cyclin B, securin and Nek2A were used as substrates. Samples were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. CaCl2 was added to initiate proteolysis.
Figure 3
The activity of Cdc20 phosphatases during the cell cycle. (A) Cdc20 substrates containing only one CDK site were phosphorylated with CDK-cyclin A and [γ-32P]ATP. The substrates (S50, T64, T68, T79 and S114) were added individually into CSF extract released by the addition of CaCl2. Release of 32PO4 was measured at intervals after addition of CaCl2. The first time point is before CaCl2 addition (−1.5 min) and the 0-min time point is immediately after CaCl2 addition. Error bars, s.e.m. from three independent experiments. (B) Phosphatase assays using Cdc20 substrates S50, T64, T68 and T79 were performed after addition of CaCl2 to CSF extract in the presence (red circle) or absence (black circle) of OA (2.5 μM). At 60 min, non-degradable cyclin B (400 nM cycBΔ167) was added to the interphase egg extracts and individual Cdc20 phosphatase assays were continuously performed. Error bars, s.e.m. from three independent experiments. (C) PP2A was removed from anaphase egg extracts by immunodepletion using anti-PP2A A subunit mAb 6F9. Cdc20 T64, T68 and T79 phosphatase activities were measured in mock- or PP2A-depleted extracts. The activities are expressed as a percentage of the phosphatase activity of the untreated anaphase extracts (white bar). Error bars, s.e.m. from three independent experiments. (D) PP2A depletion was confirmed by immunoblotting with anti-PP2A catalytic subunit antibody. Cdc2 was used to normalize the quantification. (E) Destruction of 35S-labelled cyclin B (fission yeast Cdc13) was examined in mock- or PP2A-depleted anaphase extracts. (F) Quantification of (E). Error bars, s.e.m. from three independent experiments.
Figure 4
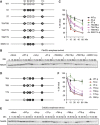
Dephosphorylation of threonine residues is required for the activation of the APC/C. (A) The diagram shows the different phosphorylation site mutants of Cdc20 (N159) used. (B) Phosphorylation of 3T (T64/68/79) largely inhibits the activation role of Cdc20. Destruction of 35S-labelled Nek2A was examined in Cdc20-depleted anaphase extracts in the presence of OA (2.5 μM) after purified N159 proteins phosphorylated by recombinant CDK were added to the extracts. Samples were taken at the indicated time points after N159 addition and analysed by SDS–PAGE and autoradiography. OA was added to prevent dephosphorylation of N159 during the experiment. (C) Quantification of the destruction assay of (B). Error bars, s.e.m. from four independent experiments. (D) The diagram shows single site mutants of N159 used. (E) The same as (B), but single site mutants of N159 were used. (F) Quantification of the destruction assay of (E). Error bars, s.e.m. from five independent experiments.
Figure 5
Threonine 79 on Cdc20 bound to the APC/C is dephosphorylated in anaphase. (A) Left panel: Overphosphorylation of Cdc20, but not APC/C, abolishes its activity. Destruction of 35S-labelled securin was examined in anaphase extracts in the presence of OA (2.5 μM) and _in vitro_-translated Cdc20 full length (Cdc20-FL) or its 5A mutant (5A-FL), as indicated. Untreated anaphase extracts (+mock) served as positive controls. Right panel: quantification of the destruction assay. Error bars, s.e.m. from four independent experiments. (B) T79 on Cdc20 bound to the APC/C is barely phosphorylated. The APC/C was purified from 50 μl of anaphase egg extracts using anti-Apc3 mAb AF3.1 and analysed by immunoblotting with anti-Cdc20 mAb (BA8) and anti-p-T79 mAb (BT2.1). In the same gel, indicated amounts of anaphase egg extracts were run and analysed under the same conditions. (C) Anaphase extracts in the presence of mock, p27 (2 μM) or OA (2.5 μM) were examined by immunoblotting with anti-Apc3, pT79 (BT2.1) or anti-Cdc2 antibodies. (D) CaCl2 was added to release CSF-arrested extracts and initiate anaphase. Apc3/Cdc27, pT79, Cdc20 and cyclin B were monitored by immunoblotting. (E) Fresh ‘cycling extract’ was treated with Dynabeads-Protein A conjugated with anti-Cdc20 antibodies. Then, to a Cdc20-depleted extract, Cdc20-FL or 5A-FL was added back to give approximately the same levels as endogenous Cdc20. These extracts were incubated at 23°C to monitor progress through the cell cycle. Samples were taken at 10 min intervals for immunoblotting of Apc3/Cdc27, pT79 (BT2.1), Cdc20, cyclin B2 and Cdc2. Untreated cycling extract (mock) and the Cdc20-depleted extract served as positive and negative controls, respectively.
Figure 6
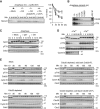
Apc6 and Apc8 associate with the activation domain of Cdc20. (A) A schematic diagram shows GST-fused Cdc20-N159 (WT) and its variants. The white box indicates an intact C-box motif whereas the black box indicates a mutated C-box motif. (B) Dephosphorylation-driven binding is independent of the C-box. The purified GST-N159 WT, ΔCb, 5A or 5A-ΔCb was bound to GSH Sepharose and incubated with CSF extract at 23°C for 20 min. The amounts of bound APC/C were analysed by immunoblotting. 50% input (lane 1) and APC/C bound to mock beads (lane 6) are also shown. The asterisk indicates a non-specific band. (C) Apc6 binds dephosphorylated Cdc20. Purified GST-N159-5A or 5E was bound to GSH Sepharose and incubated with Apc6 (left panel) or Apc8 (right panel)-expressing insect cell lysates. Co-purified Apc6 or Apc8 with GSH Sepharose-absorbed fusion proteins were monitored by immunoblotting. (D) Apc8 binds the C-box. Purified GST-N159 WT, ΔCb, 5A or 5A-ΔCb was incubated with Flag–Apc8-overexpressed insect cell lysates and Apc8 was isolated by anti-Flag IP. Co-purified N159 was monitored by immunoblotting. (E) A point mutation in Apc8 is sufficient to reduce the interaction with the C-box. Same as (D), but insect cell lysates expressing Flag–Apc8 WT, N321A mutation or mock (vector) were used. Flag–Apc8 was isolated and bound proteins were investigated. (F) The C-box is essential to activate the APC/C. Purified N159 WT or its mutant (ΔCb, 5A or 5A-ΔCb) was added to Cdc20-depleted CSF extracts and destruction of Nek2A and cyclin B (fission yeast Cdc13) was examined. Samples were taken at the indicated time points after addition of CaCl2 and analysed by SDS–PAGE and autoradiography.
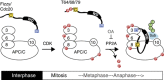
Figure 7
A model for phosphorylation-regulated APC/C activation. In interphase, both Cdc20 and the APC/C are unphosphorylated, and the APC/C is barely active. Upon entry into mitosis, CDK phosphorylates both the APC/C and Cdc20. Phosphorylation of APC/C increases its affinity for Cdc20 whereas phosphorylation of Cdc20 at T64/T68/T79 decreases its affinity to the APC/C. Thus, N-terminal phosphorylation of Cdc20 must be kept constantly in check during mitosis by phosphatase(s). At the metaphase–anaphase transition, T64/68/79 dephosphorylation of Cdc20 induces its binding to Apc6 subunit that ensures the association between the C-box and Apc8 and the subsequent activation of the APC/C. If PP2A is inhibited by OA, then the hyper-phosphorylated Cdc20 has little or no affinity for the APC/C and thus the C-box fails to activate APC/C-mediated ubiquitylation. Both CDK and PP2A are required for the activation of Cdc20-APC/C. For details, see text.
Similar articles
- A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment.
Kimata Y, Baxter JE, Fry AM, Yamano H. Kimata Y, et al. Mol Cell. 2008 Nov 21;32(4):576-83. doi: 10.1016/j.molcel.2008.09.023. Mol Cell. 2008. PMID: 19026787 - Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14.
Jaspersen SL, Charles JF, Morgan DO. Jaspersen SL, et al. Curr Biol. 1999 Mar 11;9(5):227-36. doi: 10.1016/s0960-9822(99)80111-0. Curr Biol. 1999. PMID: 10074450 - Role of phosphorylation of Cdc20 in the regulation of the action of APC/C in mitosis.
Shevah-Sitry D, Miniowitz-Shemtov S, Teichner A, Kaisari S, Hershko A. Shevah-Sitry D, et al. Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2210367119. doi: 10.1073/pnas.2210367119. Epub 2022 Aug 24. Proc Natl Acad Sci U S A. 2022. PMID: 36001690 Free PMC article. - Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex.
Kraft C, Gmachl M, Peters JM. Kraft C, et al. Methods. 2006 Jan;38(1):39-51. doi: 10.1016/j.ymeth.2005.07.005. Methods. 2006. PMID: 16343932 Review. - Regulation of APC/C activators in mitosis and meiosis.
Pesin JA, Orr-Weaver TL. Pesin JA, et al. Annu Rev Cell Dev Biol. 2008;24:475-99. doi: 10.1146/annurev.cellbio.041408.115949. Annu Rev Cell Dev Biol. 2008. PMID: 18598214 Free PMC article. Review.
Cited by
- The end of mitosis from a phosphatase perspective.
Visconti R, Palazzo L, Pepe A, Della Monica R, Grieco D. Visconti R, et al. Cell Cycle. 2013 Jan 1;12(1):17-9. doi: 10.4161/cc.22875. Cell Cycle. 2013. PMID: 23255109 Free PMC article. - Panta rhei: the APC/C at steady state.
Primorac I, Musacchio A. Primorac I, et al. J Cell Biol. 2013 Apr 15;201(2):177-89. doi: 10.1083/jcb.201301130. J Cell Biol. 2013. PMID: 23589490 Free PMC article. Review. - Negative regulation of APC/C activation by MAPK-mediated attenuation of Cdc20Slp1 under stress.
Sun L, Chen X, Song C, Shi W, Liu L, Bai S, Wang X, Chen J, Jiang C, Wang SM, Luo ZQ, Wang R, Wang Y, Jin QW. Sun L, et al. Elife. 2024 Oct 16;13:RP97896. doi: 10.7554/eLife.97896. Elife. 2024. PMID: 39412391 Free PMC article. - Kinetochores accelerate or delay APC/C activation by directing Cdc20 to opposing fates.
Kim T, Lara-Gonzalez P, Prevo B, Meitinger F, Cheerambathur DK, Oegema K, Desai A. Kim T, et al. Genes Dev. 2017 Jun 1;31(11):1089-1094. doi: 10.1101/gad.302067.117. Epub 2017 Jul 11. Genes Dev. 2017. PMID: 28698300 Free PMC article. - Aneuploidy in Cancer and Aging.
Naylor RM, van Deursen JM. Naylor RM, et al. Annu Rev Genet. 2016 Nov 23;50:45-66. doi: 10.1146/annurev-genet-120215-035303. Annu Rev Genet. 2016. PMID: 27893964 Free PMC article. Review.
References
- Barford D (2010) Structure, function and mechanism of the anaphase promoting complex (APC/C). Q Rev Biophys 44: 1–38 - PubMed
- Burton JL, Tsakraklides V, Solomon MJ (2005) Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol Cell 18: 533–542 - PubMed
- Chung E, Chen RH (2003) Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol 5: 748–753 - PubMed
- Ciliberto A, Lukacs A, Toth A, Tyson JJ, Novak B (2005) Rewiring the exit from mitosis. Cell Cycle 4: 1107–1112 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases