Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers - PubMed (original) (raw)
Review
Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers
Crislyn D'Souza-Schorey et al. Genes Dev. 2012.
Abstract
Recent advances in the study of tumor-derived microvesicles reveal new insights into the cellular basis of disease progression and the potential to translate this knowledge into innovative approaches for cancer diagnostics and personalized therapy. Tumor-derived microvesicles are heterogeneous membrane-bound sacs that are shed from the surfaces of tumor cells into the extracellular environment. They have been thought to deposit paracrine information and create paths of least resistance, as well as be taken up by cells in the tumor microenvironment to modulate the molecular makeup and behavior of recipient cells. The complexity of their bioactive cargo-which includes proteins, RNA, microRNA, and DNA-suggests multipronged mechanisms by which microvesicles can condition the extracellular milieu to facilitate disease progression. The formation of these shed vesicles likely involves both a redistribution of surface lipids and the vertical trafficking of cargo to sites of microvesicle biogenesis at the cell surface. Current research also suggests that molecular profiling of these structures could unleash their potential as circulating biomarkers as well as platforms for personalized medicine. Thus, new and improved strategies for microvesicle identification, isolation, and capture will have marked implications in point-of-care diagnostics for cancer patients.
Figures
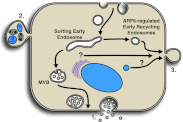
Figure 1.
Types of membrane vesicles released extracellularly by tumor cells. (1) Exosomes are formed and sequestered in preformed MVBs and released upon fusion of the MVB-limiting membrane with the plasma membrane. (2) Apoptotic bodies are formed by the random blebbing of the plasma membrane. Fragmented DNA and cytoplasmic organelles are packaged indiscriminately into these blebs. (3) TMVs contain selectively sorted cargo and form by the outward blebbing and fission of the plasma membrane. Nascent microvesicles at the cell surface may be a convergence point for multiple membrane trafficking pathways, including ARF6-regulated early endosome recycling, which directs specialized cargo to these sites of biogenesis.
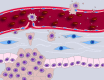
Figure 2.
TMV-mediated modulation of the tumor microenvironment. Accruing literature suggests that shed TMVs can condition the stromal microenvironment to promote angiogenesis, evasion of the immune response, tumor invasion, and, potentially, metastasis. TMVs released from tumor cells (brown) can be taken up by cells in the tumor microenvironment, such as carcinoma-associated fibroblasts (blue), with consequences for target cell behavior. They can also interact with the extracellular matrix by depositing paracrine information or facilitating matrix degradation, thereby creating paths of least resistance.
Similar articles
- Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations.
Santana SM, Antonyak MA, Cerione RA, Kirby BJ. Santana SM, et al. Biomed Microdevices. 2014 Dec;16(6):869-77. doi: 10.1007/s10544-014-9891-z. Biomed Microdevices. 2014. PMID: 25342569 Free PMC article. - Biology and biogenesis of shed microvesicles.
Tricarico C, Clancy J, D'Souza-Schorey C. Tricarico C, et al. Small GTPases. 2017 Oct 2;8(4):220-232. doi: 10.1080/21541248.2016.1215283. Epub 2016 Aug 5. Small GTPases. 2017. PMID: 27494381 Free PMC article. Review. - Microvesicles in Cancer: Small Size, Large Potential.
Menck K, Sivaloganathan S, Bleckmann A, Binder C. Menck K, et al. Int J Mol Sci. 2020 Jul 28;21(15):5373. doi: 10.3390/ijms21155373. Int J Mol Sci. 2020. PMID: 32731639 Free PMC article. Review. - Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy?
Martellucci S, Orefice NS, Angelucci A, Luce A, Caraglia M, Zappavigna S. Martellucci S, et al. Int J Mol Sci. 2020 Sep 4;21(18):6486. doi: 10.3390/ijms21186486. Int J Mol Sci. 2020. PMID: 32899898 Free PMC article. Review. - Tumor-cell-derived microvesicles as carriers of molecular information in cancer.
Martins VR, Dias MS, Hainaut P. Martins VR, et al. Curr Opin Oncol. 2013 Jan;25(1):66-75. doi: 10.1097/CCO.0b013e32835b7c81. Curr Opin Oncol. 2013. PMID: 23165142 Review.
Cited by
- Circulating extracellular vesicles in cancer diagnosis and monitoring: an appraisal of clinical potential.
Choi DS, Lee J, Go G, Kim YK, Gho YS. Choi DS, et al. Mol Diagn Ther. 2013 Oct;17(5):265-71. doi: 10.1007/s40291-013-0042-7. Mol Diagn Ther. 2013. PMID: 23729224 - Exosomes: New Insights into the Pathogenesis of Metabolic Syndrome.
Wang N, Li J, Hu Z, Ngowi EE, Yan B, Qiao A. Wang N, et al. Biology (Basel). 2023 Dec 1;12(12):1480. doi: 10.3390/biology12121480. Biology (Basel). 2023. PMID: 38132306 Free PMC article. Review. - Characterization of TCP-1 probes for molecular imaging of colon cancer.
Liu Z, Gray BD, Barber C, Bernas M, Cai M, Furenlid LR, Rouse A, Patel C, Banerjee B, Liang R, Gmitro AF, Witte MH, Pak KY, Woolfenden JM. Liu Z, et al. J Control Release. 2016 Oct 10;239:223-30. doi: 10.1016/j.jconrel.2016.08.033. Epub 2016 Aug 26. J Control Release. 2016. PMID: 27574992 Free PMC article. - Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates.
Luo Y, Li Z, Wang X, Wang J, Duan X, Li R, Peng Y, Ye Q, He Y. Luo Y, et al. Front Bioeng Biotechnol. 2022 Sep 16;10:1016833. doi: 10.3389/fbioe.2022.1016833. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185445 Free PMC article. Review. - Role of Intracellular and Extracellular MicroRNA-92a in Colorectal Cancer.
Yamada N, Nakagawa Y, Tsujimura N, Kumazaki M, Noguchi S, Mori T, Hirata I, Maruo K, Akao Y. Yamada N, et al. Transl Oncol. 2013 Aug 1;6(4):482-92. doi: 10.1593/tlo.13280. Print 2013 Aug. Transl Oncol. 2013. PMID: 23908691 Free PMC article.
References
- Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ, Mor G 2003. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res 63: 5573–5581 - PubMed
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10: 619–624 - PubMed
- Al-Nedawi K, Meehan B, Rak J 2009b. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle 8: 2014–2018 - PubMed
- Andersen KB, Levinsen S, Svendsen WE, Okkels F 2009. A generalized theoretical model for ‘continuous particle separation in a microchannel having asymmetrically arranged multiple branches.’ Lab Chip 9: 1638–1639 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources